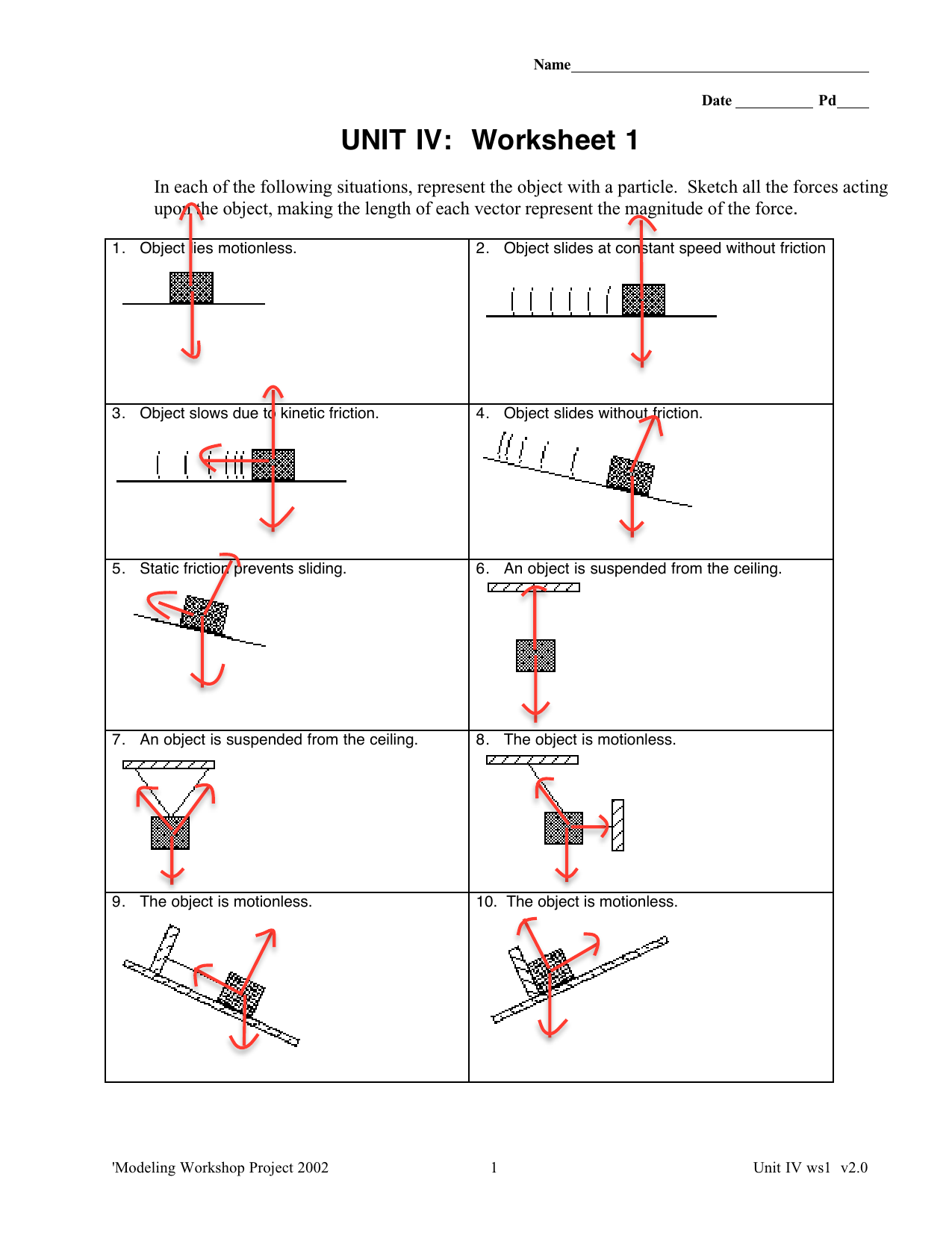
Making Dilutions Worksheet

Understanding Dilutions: A Step-by-Step Guide
Dilutions are a crucial concept in chemistry, biology, and various scientific fields. It involves reducing the concentration of a substance by adding a solvent, which can be water or any other liquid. In this article, we will explore the concept of dilutions, how to calculate dilutions, and provide a worksheet to help you practice.
What is a Dilution?
A dilution is a process of reducing the concentration of a substance by adding a solvent. The resulting solution has a lower concentration of the substance than the original solution. Dilutions are often used in scientific experiments, medical treatments, and industrial processes.
Types of Dilutions
There are two main types of dilutions:
- Serial Dilution: A serial dilution involves making multiple dilutions of a solution, where each subsequent dilution is made from the previous one.
- Parallel Dilution: A parallel dilution involves making multiple dilutions of a solution at the same time, using the same stock solution.
Calculating Dilutions
To calculate a dilution, you need to know the initial concentration of the substance, the final concentration, and the volume of the solvent added. The formula for calculating a dilution is:
Dilution Factor (DF) = Initial Concentration (IC) / Final Concentration (FC)
Volume of Solvent Added (V) = (DF - 1) x Initial Volume (IV)
Where:
- IC is the initial concentration of the substance
- FC is the final concentration of the substance
- DF is the dilution factor
- V is the volume of the solvent added
- IV is the initial volume of the solution
How to Make a Dilution
Here’s a step-by-step guide to making a dilution:
- Prepare the stock solution: Make sure you have the stock solution with the initial concentration.
- Determine the dilution factor: Calculate the dilution factor using the formula above.
- Measure the initial volume: Measure the initial volume of the stock solution.
- Add the solvent: Add the calculated volume of solvent to the initial volume of the stock solution.
- Mix the solution: Mix the solution thoroughly to ensure uniform distribution of the substance.
🔬 Note: Always use a pipette to measure the volumes accurately, and mix the solution well to avoid any errors.
Dilutions Worksheet
Here’s a worksheet to help you practice calculating dilutions:

| Initial Concentration (IC) | Final Concentration (FC) | Initial Volume (IV) | Volume of Solvent Added (V) |
|---|---|---|---|
| 10 M | 2 M | 5 mL | ? |
| 5 M | 1 M | 10 mL | ? |
| 20 M | 5 M | 2 mL | ? |
| 15 M | 3 M | 8 mL | ? |
| 8 M | 2 M | 12 mL | ? |
Answers:
| Initial Concentration (IC) | Final Concentration (FC) | Initial Volume (IV) | Volume of Solvent Added (V) |
|---|---|---|---|
| 10 M | 2 M | 5 mL | 15 mL |
| 5 M | 1 M | 10 mL | 40 mL |
| 20 M | 5 M | 2 mL | 6 mL |
| 15 M | 3 M | 8 mL | 24 mL |
| 8 M | 2 M | 12 mL | 48 mL |
Conclusion
Dilutions are an essential concept in various scientific fields, and understanding how to calculate and make dilutions is crucial for accurate results. By following the steps outlined in this article and practicing with the worksheet, you’ll become proficient in making dilutions and applying this concept to real-world problems.
What is the purpose of a dilution?
+The purpose of a dilution is to reduce the concentration of a substance by adding a solvent, which can be used in various scientific experiments, medical treatments, and industrial processes.
What is the difference between a serial dilution and a parallel dilution?
+A serial dilution involves making multiple dilutions of a solution, where each subsequent dilution is made from the previous one. A parallel dilution involves making multiple dilutions of a solution at the same time, using the same stock solution.
How do I calculate the volume of solvent added in a dilution?
+The volume of solvent added can be calculated using the formula: V = (DF - 1) x IV, where V is the volume of the solvent added, DF is the dilution factor, and IV is the initial volume of the solution.
Related Terms:
- Dilutions Worksheet answer Key
- Dilution problems Worksheet with answers