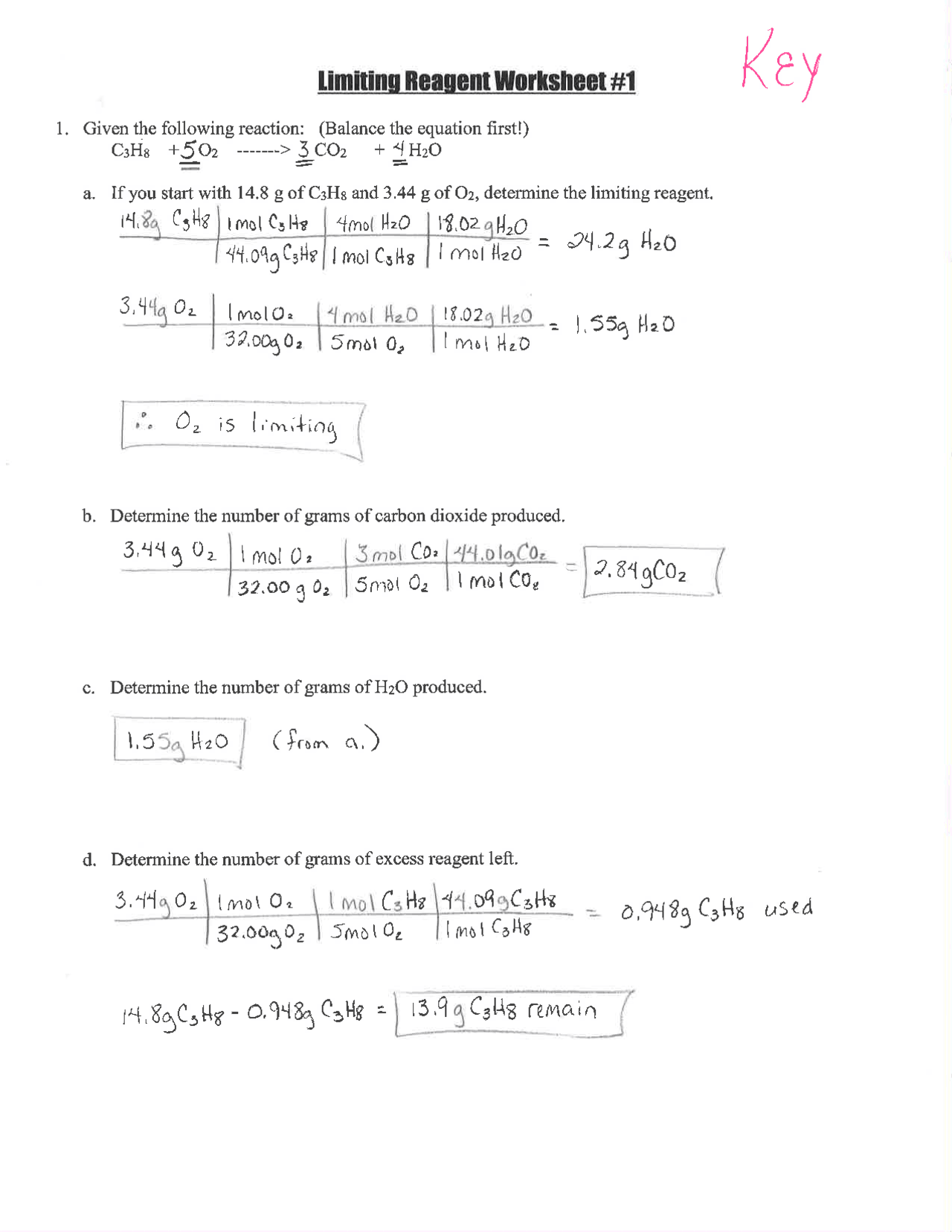
Limiting Reagent Worksheet Answer Key
Understanding Limiting Reagents: A Comprehensive Guide
In chemistry, a limiting reagent is a reactant that determines the amount of product that can be formed in a chemical reaction. This concept is crucial in understanding the stoichiometry of chemical reactions and predicting the outcome of a reaction. In this article, we will delve into the world of limiting reagents, explore the concept in depth, and provide a worksheet answer key to help you practice.
What is a Limiting Reagent?
A limiting reagent is a reactant that is completely consumed in a chemical reaction, thereby determining the amount of product that can be formed. The limiting reagent is usually the reactant that is present in the smallest stoichiometric amount. In other words, it is the reactant that will be used up first, and once it is depleted, the reaction will stop.
How to Identify the Limiting Reagent
To identify the limiting reagent, you need to compare the mole ratio of the reactants to the coefficients in the balanced equation. The reactant with the smallest mole ratio is usually the limiting reagent. Here’s a step-by-step guide to identifying the limiting reagent:
- Write the balanced equation for the reaction.
- Calculate the mole ratio of each reactant.
- Compare the mole ratio to the coefficients in the balanced equation.
- Identify the reactant with the smallest mole ratio.
Limiting Reagent Worksheet
Here’s a worksheet to help you practice identifying the limiting reagent:
Reaction 1:
2Na + Cl2 → 2NaCl
Given:
- 10 moles of Na
- 5 moles of Cl2
Question: Which reactant is the limiting reagent?
Answer: Cl2 (because the mole ratio of Cl2 is 5, which is less than the coefficient of 2 in the balanced equation)
Reaction 2:
Ca + 2HCl → CaCl2 + H2
Given:
- 20 moles of Ca
- 15 moles of HCl
Question: Which reactant is the limiting reagent?
Answer: HCl (because the mole ratio of HCl is 15, which is less than twice the coefficient of Ca in the balanced equation)
Reaction 3:
2Al + Fe2O3 → 2Fe + Al2O3
Given:
- 30 moles of Al
- 20 moles of Fe2O3
Question: Which reactant is the limiting reagent?
Answer: Fe2O3 (because the mole ratio of Fe2O3 is 20, which is less than twice the coefficient of Al in the balanced equation)
Notes
🔍 Note: The limiting reagent is not always the reactant with the smallest amount of substance. It's the reactant that determines the amount of product that can be formed.
📝 Note: Make sure to balance the equation and calculate the mole ratio of each reactant before identifying the limiting reagent.
Why is the Limiting Reagent Important?
The limiting reagent is crucial in understanding the stoichiometry of chemical reactions. It helps predict the amount of product that can be formed and determines the yield of the reaction. In industrial applications, understanding the limiting reagent is essential for optimizing reaction conditions and maximizing product yield.
Conclusion
In conclusion, the limiting reagent is a fundamental concept in chemistry that determines the amount of product that can be formed in a chemical reaction. By understanding the concept of limiting reagents, you can predict the outcome of a reaction and optimize reaction conditions to maximize product yield. Practice identifying the limiting reagent with the worksheet provided, and you’ll become proficient in no time.
What is the definition of a limiting reagent?
+A limiting reagent is a reactant that determines the amount of product that can be formed in a chemical reaction.
How do I identify the limiting reagent?
+Compare the mole ratio of each reactant to the coefficients in the balanced equation. The reactant with the smallest mole ratio is usually the limiting reagent.
Why is the limiting reagent important?
+The limiting reagent helps predict the amount of product that can be formed and determines the yield of the reaction.
Related Terms:
- Limiting reagent Worksheet answers
- Limiting reagent Worksheet 2
- Limiting reagent Worksheet #1
- Limiting reagents 1 answers