Mastering Limiting Reagent and Percent Yield Calculations
Understanding Limiting Reagent and Percent Yield Calculations
In chemical reactions, it’s essential to understand the concept of limiting reagent and percent yield calculations. These calculations help chemists determine the maximum amount of product that can be obtained from a reaction, as well as the efficiency of the reaction. In this article, we’ll explore the concept of limiting reagent and percent yield calculations, and provide examples to illustrate how to perform these calculations.
What is Limiting Reagent?
The limiting reagent, also known as the limiting reactant, is the reactant that determines the amount of product that can be formed in a chemical reaction. This occurs when one reactant is present in a lesser amount than the other reactants, and the reaction cannot proceed without it. The limiting reagent is usually the reactant that is consumed first, and the reaction stops when it is depleted.
📝 Note: The limiting reagent is not always the reactant with the smallest amount, but rather the reactant that is required in the smallest amount according to the balanced chemical equation.
How to Determine the Limiting Reagent
To determine the limiting reagent, we need to calculate the number of moles of each reactant and compare them to the coefficients in the balanced chemical equation. Here are the steps:
- Write the balanced chemical equation for the reaction.
- Calculate the number of moles of each reactant using the formula: moles = mass / molar mass.
- Compare the number of moles of each reactant to the coefficients in the balanced chemical equation.
- Identify the reactant with the smallest mole ratio compared to the coefficients in the balanced equation.
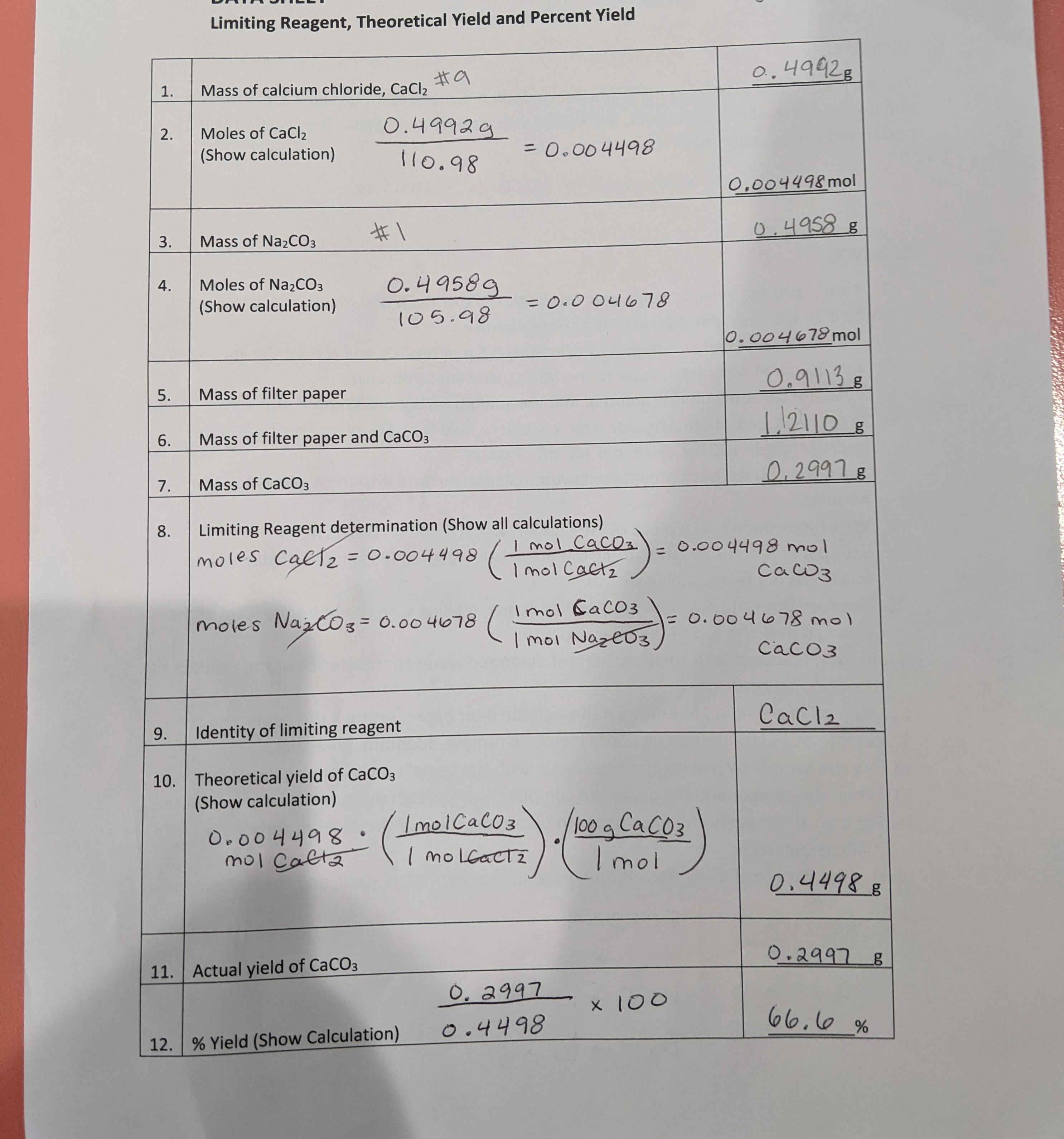
Example: Determining the Limiting Reagent
Suppose we have the following reaction:
2A + 3B → 2C
We are given 10 grams of A and 15 grams of B. The molar masses of A and B are 20 g/mol and 30 g/mol, respectively.
First, we calculate the number of moles of each reactant:
moles A = 10 g / 20 g/mol = 0.5 mol moles B = 15 g / 30 g/mol = 0.5 mol
Next, we compare the number of moles of each reactant to the coefficients in the balanced equation:
A: 0.5 mol / 2 = 0.25 B: 0.5 mol / 3 = 0.17
Since B has the smallest mole ratio, it is the limiting reagent.
What is Percent Yield?
Percent yield is a measure of the efficiency of a chemical reaction. It is calculated by dividing the actual yield of a product by the theoretical yield and multiplying by 100. The theoretical yield is the maximum amount of product that can be obtained from a reaction, based on the balanced chemical equation.
How to Calculate Percent Yield
To calculate percent yield, we need to know the actual yield of the product and the theoretical yield. Here are the steps:
- Calculate the theoretical yield using the formula: theoretical yield = number of moles of limiting reagent x coefficient of product in balanced equation x molar mass of product.
- Measure the actual yield of the product.
- Calculate the percent yield using the formula: percent yield = (actual yield / theoretical yield) x 100.
Example: Calculating Percent Yield
Suppose we have the following reaction:
2A + 3B → 2C
We are given 10 grams of A and 15 grams of B. The molar masses of A and B are 20 g/mol and 30 g/mol, respectively. We know that B is the limiting reagent.
First, we calculate the theoretical yield:
theoretical yield = 0.5 mol B x 2 x 40 g/mol = 40 g
Next, we measure the actual yield: 30 g
Finally, we calculate the percent yield:
percent yield = (30 g / 40 g) x 100 = 75%
This means that the reaction is 75% efficient.
Importance of Limiting Reagent and Percent Yield Calculations
Limiting reagent and percent yield calculations are essential in chemical reactions. They help chemists:
- Determine the maximum amount of product that can be obtained from a reaction.
- Identify the reactant that limits the reaction.
- Measure the efficiency of a reaction.
- Optimize reaction conditions to achieve higher yields.
Common Mistakes to Avoid
When performing limiting reagent and percent yield calculations, it’s essential to avoid the following common mistakes:
- Not balancing the chemical equation.
- Not calculating the number of moles of each reactant correctly.
- Not identifying the limiting reagent correctly.
- Not measuring the actual yield correctly.
By understanding limiting reagent and percent yield calculations, chemists can optimize reaction conditions, improve yields, and ensure the efficiency of chemical reactions.
In the next section, we’ll provide some frequently asked questions about limiting reagent and percent yield calculations.
What is the difference between limiting reagent and excess reagent?
+The limiting reagent is the reactant that determines the amount of product that can be formed in a chemical reaction, while the excess reagent is the reactant that is present in excess and does not limit the reaction.
How do you calculate the theoretical yield of a reaction?
+The theoretical yield is calculated by multiplying the number of moles of the limiting reagent by the coefficient of the product in the balanced chemical equation and the molar mass of the product.
What is the significance of percent yield in chemical reactions?
+Percent yield is a measure of the efficiency of a chemical reaction. It helps chemists determine the actual yield of a product compared to the theoretical yield and identify areas for improvement.