7 Steps to Master Limiting Reactant and Percent Yield
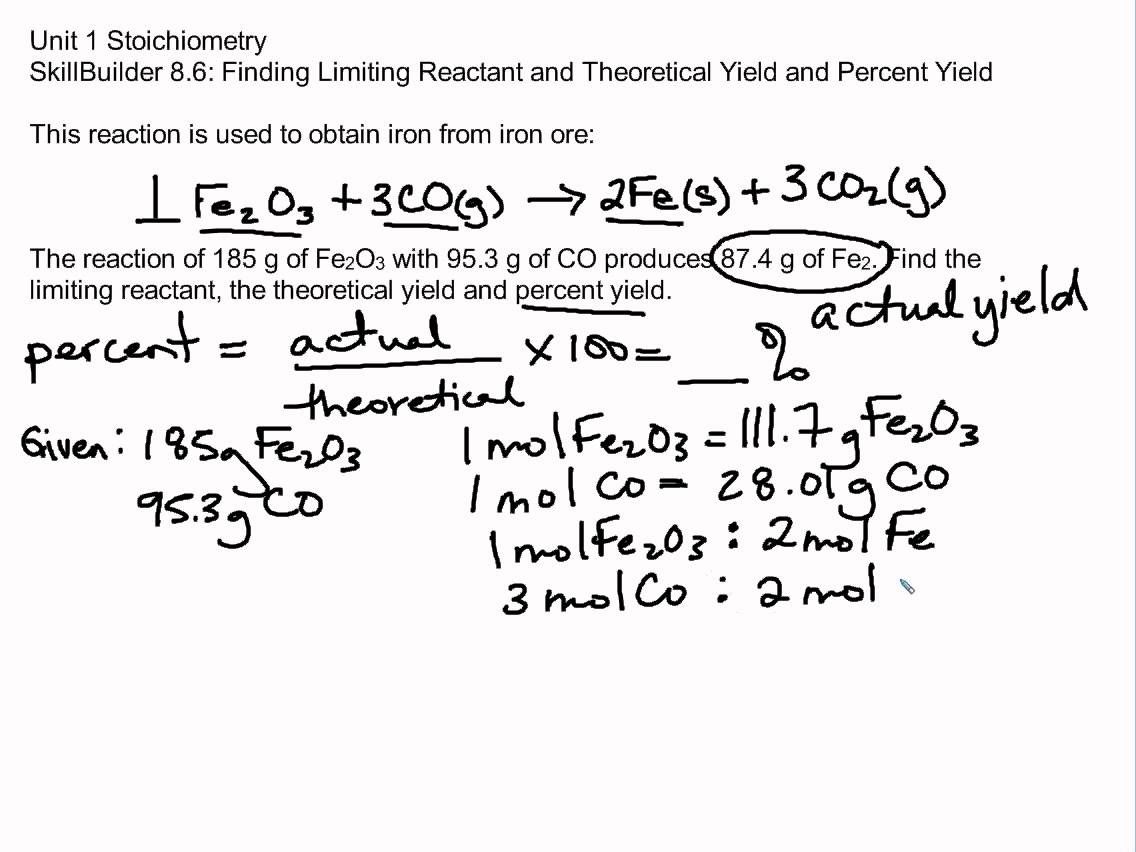
Mastering limiting reactant and percent yield is crucial for any student of chemistry. These concepts are fundamental to understanding chemical reactions and are used extensively in various fields, including pharmacy, chemical engineering, and materials science. In this article, we will break down the steps to master limiting reactant and percent yield, providing a comprehensive guide for students to excel in these areas.
Step 1: Understand the Concept of Limiting Reactant
The limiting reactant is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product formed. It is essential to identify the limiting reactant in a reaction to calculate the maximum amount of product that can be formed.
🔍 Note: The limiting reactant is not always the reactant with the smallest amount. It depends on the stoichiometry of the reaction.
Step 2: Balance Chemical Equations
Balancing chemical equations is crucial in determining the limiting reactant. A balanced equation ensures that the number of atoms of each element is the same on both the reactant and product sides. To balance an equation, we need to add coefficients (numbers in front of formulas of reactants or products) to ensure the number of atoms of each element is equal.
Example:
Unbalanced equation: Ca + O2 → CaO Balanced equation: 2Ca + O2 → 2CaO
Step 3: Calculate the Number of Moles of Each Reactant
To determine the limiting reactant, we need to calculate the number of moles of each reactant. This can be done using the molar mass of each reactant and the given mass.
📝 Note: The molar mass of an element is the sum of the atomic masses of its constituent atoms.
Step 4: Determine the Limiting Reactant
Once we have calculated the number of moles of each reactant, we can determine the limiting reactant by comparing the mole ratio of each reactant to the coefficients in the balanced equation.
Example:
Consider the reaction: 2A + 3B → 2C
| Reactant | Moles | Coefficient |
|---|---|---|
| A | 2 | 2 |
| B | 6 | 3 |
In this case, reactant A is the limiting reactant since it has a lower mole ratio compared to its coefficient.
Step 5: Calculate the Theoretical Yield
The theoretical yield is the maximum amount of product that can be formed in a reaction. It can be calculated using the number of moles of the limiting reactant and the balanced equation.
Example:
Consider the reaction: 2A + 3B → 2C
If reactant A is the limiting reactant with 2 moles, the theoretical yield of product C can be calculated as:
Theoretical yield = (2 moles A) x (2 moles C / 2 moles A) = 2 moles C
Step 6: Calculate the Percent Yield
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage.
📊 Note: The actual yield is the amount of product obtained in the reaction.
Example:
Consider the reaction: 2A + 3B → 2C
If the actual yield of product C is 1.8 moles, the percent yield can be calculated as:
Percent yield = (1.8 moles C / 2 moles C) x 100% = 90%
Step 7: Practice and Apply
Mastering limiting reactant and percent yield requires practice and application. Students should practice solving problems and apply these concepts to real-world scenarios.
📚 Note: There are many online resources and textbooks that provide practice problems and examples to help students master limiting reactant and percent yield.
In summary, mastering limiting reactant and percent yield requires a deep understanding of chemical reactions, balancing equations, and calculating mole ratios. By following these steps, students can develop a strong foundation in these concepts and excel in their chemistry studies.
What is the limiting reactant in a chemical reaction?
+The limiting reactant is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product formed.
How do I calculate the theoretical yield of a reaction?
+The theoretical yield can be calculated using the number of moles of the limiting reactant and the balanced equation.
What is the difference between actual yield and percent yield?
+The actual yield is the amount of product obtained in the reaction, while the percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage.