5 Ways to Master Limiting Reactant and Percent Yield

Understanding Limiting Reactant and Percent Yield
In chemistry, understanding the concepts of limiting reactant and percent yield is crucial for any student or professional. The limiting reactant is the reactant that determines the amount of product that can be formed in a chemical reaction, while percent yield is the ratio of the actual yield to the theoretical yield. Mastering these concepts can help you predict the outcome of a reaction, optimize reaction conditions, and troubleshoot any issues that may arise.
What is Limiting Reactant?
The limiting reactant is the reactant that is completely consumed in a reaction, thereby limiting the amount of product that can be formed. It is also known as the limiting reagent or limiting agent. The limiting reactant can be identified by comparing the mole ratio of the reactants to the mole ratio of the products in the balanced chemical equation.
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 → 2H2O
In this reaction, the mole ratio of H2 to O2 is 2:1. If we have 2 moles of H2 and 1 mole of O2, then H2 is the limiting reactant because it will be completely consumed in the reaction.
What is Percent Yield?
Percent yield is a measure of the efficiency of a reaction, expressed as a percentage of the theoretical yield. The theoretical yield is the maximum amount of product that can be formed in a reaction, based on the limiting reactant. The actual yield is the amount of product that is actually obtained.
Percent yield = (actual yield / theoretical yield) x 100
For example, if the theoretical yield of a reaction is 100 grams, and the actual yield is 80 grams, then the percent yield is:
Percent yield = (80 g / 100 g) x 100 = 80%
5 Ways to Master Limiting Reactant and Percent Yield
Here are five ways to master limiting reactant and percent yield:
1. Understand the Balanced Chemical Equation
To master limiting reactant and percent yield, you need to understand the balanced chemical equation. The balanced chemical equation shows the mole ratio of the reactants to the products. By analyzing the balanced equation, you can identify the limiting reactant and calculate the theoretical yield.
| Reactant | Coeficient | Product | Coeficient |
|---|---|---|---|
| H2 | 2 | H2O | 2 |
| O2 | 1 |
2. Calculate the Limiting Reactant
To calculate the limiting reactant, you need to compare the mole ratio of the reactants to the mole ratio of the products in the balanced chemical equation. You can use the following steps:
- Calculate the number of moles of each reactant
- Compare the mole ratio of the reactants to the mole ratio of the products
- Identify the reactant that is completely consumed in the reaction
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 → 2H2O
If we have 2 moles of H2 and 1 mole of O2, then H2 is the limiting reactant because it will be completely consumed in the reaction.
3. Calculate the Theoretical Yield
To calculate the theoretical yield, you need to use the limiting reactant and the balanced chemical equation. You can use the following steps:
- Identify the limiting reactant
- Calculate the number of moles of the limiting reactant
- Use the mole ratio of the limiting reactant to the product to calculate the theoretical yield
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 → 2H2O
If we have 2 moles of H2, then the theoretical yield of H2O is:
Theoretical yield = 2 moles x (2 moles H2O / 2 moles H2) = 2 moles
4. Calculate the Percent Yield
To calculate the percent yield, you need to use the actual yield and the theoretical yield. You can use the following steps:
- Calculate the actual yield
- Calculate the theoretical yield
- Use the formula: percent yield = (actual yield / theoretical yield) x 100
For example, if the theoretical yield of a reaction is 100 grams, and the actual yield is 80 grams, then the percent yield is:
Percent yield = (80 g / 100 g) x 100 = 80%
5. Practice with Sample Problems
To master limiting reactant and percent yield, you need to practice with sample problems. You can use online resources or textbooks to find sample problems. Practice problems can help you develop your skills and build your confidence.
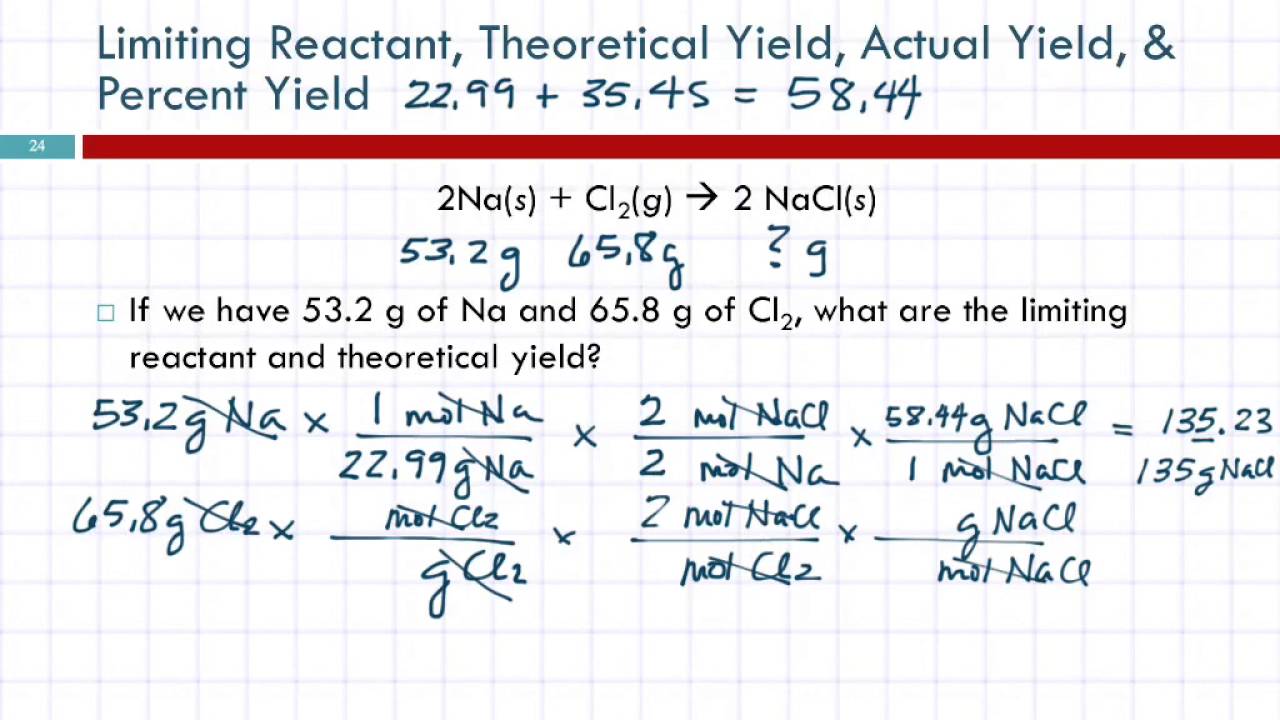
For example, consider the reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) to form sodium chloride (NaCl) and water (H2O):
NaOH + HCl → NaCl + H2O
If we have 1 mole of NaOH and 1 mole of HCl, then what is the limiting reactant and the theoretical yield of NaCl?
By practicing with sample problems, you can develop your skills and build your confidence in mastering limiting reactant and percent yield.
[💡] Note: Remember to always check your units and ensure that you are using the correct mole ratio when calculating the limiting reactant and the theoretical yield.
In conclusion, mastering limiting reactant and percent yield requires practice and a deep understanding of the balanced chemical equation. By following the five steps outlined above, you can develop your skills and build your confidence in mastering these concepts.
What is the difference between limiting reactant and excess reactant?
+The limiting reactant is the reactant that is completely consumed in a reaction, while the excess reactant is the reactant that is not completely consumed in a reaction.
How do I calculate the percent yield?
+The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100.
What is the importance of mastering limiting reactant and percent yield?
+Mastering limiting reactant and percent yield is important because it allows you to predict the outcome of a reaction, optimize reaction conditions, and troubleshoot any issues that may arise.