5 Ways to Master Lewis Structures
Understanding Lewis Structures: A Fundamental Concept in Chemistry
Lewis structures are a crucial aspect of chemistry, as they help us visualize the arrangement of electrons in a molecule. Developed by Gilbert N. Lewis in 1916, these structures have become an essential tool for chemists to predict the properties and behavior of molecules. Mastering Lewis structures is vital for any student of chemistry, and in this article, we will explore five ways to help you achieve proficiency in drawing and interpreting these structures.
1. Start with the Basics: Understanding Valence Electrons
To draw Lewis structures, you need to understand the concept of valence electrons. Valence electrons are the electrons in the outermost energy level of an atom, which participate in chemical bonding. The number of valence electrons an atom has determines its reactivity and the types of bonds it can form.
- Step 1: Identify the atoms in the molecule and determine their valence electrons.
- Step 2: Write the symbols of the atoms and indicate the number of valence electrons for each atom.
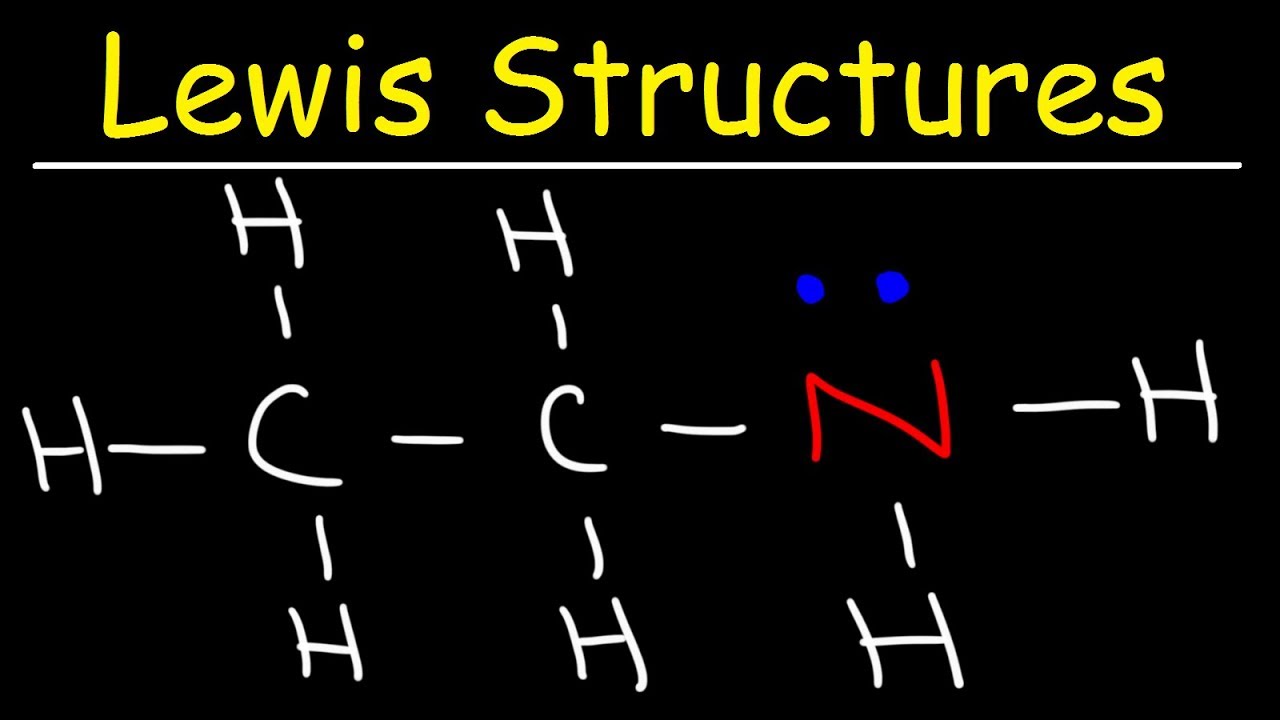
For example, let’s consider the molecule CH₄ (methane). The valence electrons for carbon © are 4, and for hydrogen (H) are 1.
💡 Note: Make sure to use the correct number of valence electrons for each atom, as this will affect the accuracy of your Lewis structure.
2. Draw the Skeleton Structure
Once you have identified the atoms and their valence electrons, you can start drawing the skeleton structure of the molecule. The skeleton structure shows the arrangement of atoms in the molecule, without considering the electrons.
- Step 1: Connect the atoms with single bonds, starting with the central atom (usually the atom with the lowest electronegativity).
- Step 2: Arrange the atoms in a way that minimizes the formal charges on each atom.
For example, in the case of CH₄, we start by connecting the carbon atom to the four hydrogen atoms with single bonds.
3. Distribute the Electrons
Now that you have the skeleton structure, you can distribute the electrons among the atoms. The goal is to minimize the formal charges on each atom and satisfy the octet rule (8 electrons in the outermost energy level).
- Step 1: Place two electrons between each pair of bonded atoms to represent the covalent bonds.
- Step 2: Distribute the remaining electrons among the atoms, starting with the central atom.
- Step 3: Check if each atom has a full octet (8 electrons) and adjust the electrons accordingly.
For example, in the case of CH₄, we place two electrons between the carbon and each hydrogen atom, and then distribute the remaining electrons among the atoms.
| Atom | Valence Electrons | Formal Charge |
|---|---|---|
| C | 4 | 0 |
| H | 1 | 0 |
| H | 1 | 0 |
| H | 1 | 0 |
| H | 1 | 0 |
4. Check the Formal Charges
Formal charges are a measure of the electronegativity difference between atoms in a molecule. A formal charge of 0 indicates that the atom has the same number of electrons as it would in its neutral state.
- Step 1: Calculate the formal charge for each atom using the formula: formal charge = valence electrons - nonbonding electrons - (bonding electrons / 2).
- Step 2: Check if each atom has a formal charge of 0. If not, adjust the electrons to minimize the formal charges.
For example, in the case of CH₄, we calculate the formal charge for each atom and adjust the electrons to ensure that each atom has a formal charge of 0.
5. Practice, Practice, Practice!
Mastering Lewis structures requires practice. Start with simple molecules and gradually move on to more complex ones.
- Step 1: Practice drawing Lewis structures for different molecules, using the steps outlined above.
- Step 2: Check your answers with online resources or textbooks to ensure accuracy.
By following these five steps and practicing regularly, you will become proficient in drawing and interpreting Lewis structures, which will help you better understand the properties and behavior of molecules.
What is the purpose of Lewis structures?
+Lewis structures help us visualize the arrangement of electrons in a molecule, which is essential for predicting the properties and behavior of molecules.
What are valence electrons?
+Valence electrons are the electrons in the outermost energy level of an atom, which participate in chemical bonding.
How do I calculate formal charges?
+Formal charge = valence electrons - nonbonding electrons - (bonding electrons / 2).