Lewis Structure Worksheet 1 Answers Key
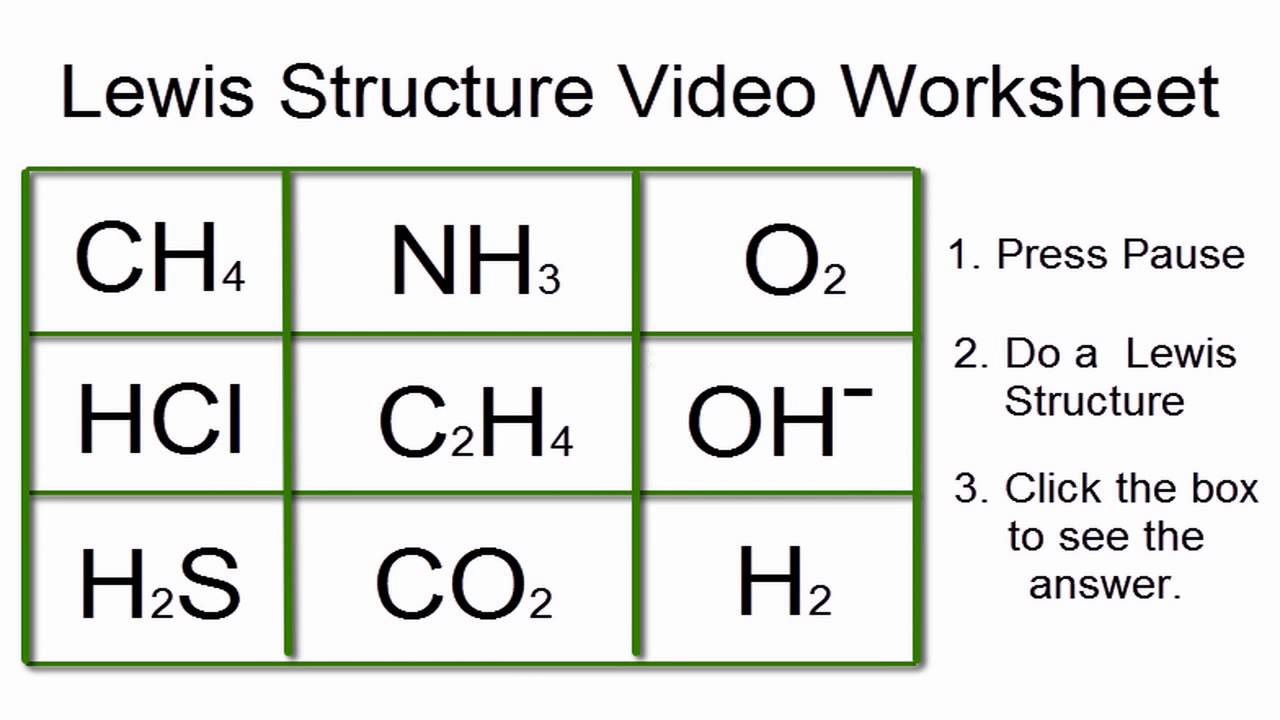
Lewis Structure Worksheet 1 Answers Key
The Lewis structure, also known as the electron dot structure, is a graphical representation of the bonding between atoms in a molecule. It is a fundamental concept in chemistry and is used to describe the arrangement of electrons in a molecule. In this answer key, we will go through the answers to Lewis Structure Worksheet 1.
What is a Lewis Structure?
A Lewis structure is a diagram that shows the bonding between atoms in a molecule. It is a two-dimensional representation of the molecule, with atoms represented by their chemical symbols and electrons represented by dots.
How to Draw a Lewis Structure
To draw a Lewis structure, follow these steps:
- Determine the total number of valence electrons in the molecule.
- Determine the central atom (usually the least electronegative atom).
- Determine the number of electrons needed to complete the octet of each atom.
- Draw single bonds between the central atom and the surrounding atoms.
- Add electrons to the surrounding atoms to complete their octets.
- Add electrons to the central atom to complete its octet.
Lewis Structure Worksheet 1 Answers
Here are the answers to Lewis Structure Worksheet 1:
Problem 1: CH4
The Lewis structure for CH4 is:
H - C - H
| |
H H
Total valence electrons: 8
Central atom: C
Electrons needed to complete octet: 4
📝 Note: The carbon atom has four single bonds to hydrogen atoms, which complete its octet.
Problem 2: NH3
The Lewis structure for NH3 is:
H - N - H
| |
H (pair of electrons)
Total valence electrons: 8
Central atom: N
Electrons needed to complete octet: 3
💡 Note: The nitrogen atom has three single bonds to hydrogen atoms and a lone pair of electrons, which complete its octet.
Problem 3: H2O
The Lewis structure for H2O is:
H - O - H
| |
(pair of electrons) (pair of electrons)
Total valence electrons: 8
Central atom: O
Electrons needed to complete octet: 2
🌊 Note: The oxygen atom has two single bonds to hydrogen atoms and two lone pairs of electrons, which complete its octet.
Conclusion
In conclusion, the Lewis structure is a powerful tool for understanding the bonding between atoms in a molecule. By following the steps outlined above, you can draw the Lewis structure for a given molecule. The answers to Lewis Structure Worksheet 1 are provided above.
What is the purpose of a Lewis structure?
+The purpose of a Lewis structure is to provide a graphical representation of the bonding between atoms in a molecule.
What is the central atom in a Lewis structure?
+The central atom is usually the least electronegative atom in the molecule.
How do you determine the number of electrons needed to complete the octet of an atom?
+The number of electrons needed to complete the octet of an atom is equal to 8 minus the number of valence electrons already present.
Related Terms:
- Lewis Structure Worksheet 2 answers
- Lewis structure practice problems
- Lewis structure practice answer key
- Worksheet Lewis structure
- Lewis structure and Geometry Worksheet