5 Ways to Master Lewis Dot Structure Practice
Understanding the Basics of Lewis Dot Structure
Lewis dot structure is a fundamental concept in chemistry that represents the valence electrons of atoms within a molecule. It is a crucial tool for chemists to visualize and understand the bonding and structure of molecules. Mastering Lewis dot structure practice is essential for students and professionals alike to excel in chemistry. In this article, we will explore five ways to master Lewis dot structure practice.
1. Familiarize Yourself with the Rules
Before practicing Lewis dot structures, it is essential to understand the rules that govern their construction. Here are the basic rules to keep in mind:
- Rule 1: The total number of valence electrons in a molecule is equal to the sum of the valence electrons of each atom.
- Rule 2: Atoms tend to gain, lose, or share electrons to achieve a full outer shell, which typically consists of eight electrons.
- Rule 3: Electrons are represented by dots, and each dot represents one electron.
- Rule 4: Bonds between atoms are represented by lines, and each line represents a pair of shared electrons.
📝 Note: It is crucial to understand these rules before practicing Lewis dot structures.
2. Practice with Simple Molecules
Start by practicing Lewis dot structures with simple molecules, such as H2, O2, and CO2. These molecules have a small number of atoms and electrons, making it easier to visualize and construct their Lewis dot structures.
- Example: Construct the Lewis dot structure for H2.
- Step 1: Determine the total number of valence electrons (2).
- Step 2: Draw the atoms and represent the electrons as dots.
- Step 3: Connect the atoms with a line, representing a shared pair of electrons.
3. Use Online Tools and Resources
There are many online tools and resources available to practice Lewis dot structures, such as:
- Online Lewis dot structure generators: These tools allow you to input the molecular formula and generate the Lewis dot structure.
- Interactive tutorials: Websites like Khan Academy and Chemistry LibreTexts offer interactive tutorials and practice exercises.
- Practice quizzes: Online quizzes and tests can help you assess your knowledge and identify areas for improvement.
4. Focus on Resonance and Exceptions
Resonance and exceptions are critical concepts in Lewis dot structure practice. Resonance occurs when a molecule has multiple Lewis dot structures that contribute to its overall structure. Exceptions refer to molecules that do not follow the usual rules for constructing Lewis dot structures.
- Example: Construct the Lewis dot structure for NO2.
- Step 1: Determine the total number of valence electrons (17).
- Step 2: Draw the atoms and represent the electrons as dots.
- Step 3: Recognize that NO2 has resonance structures, and construct the possible resonance forms.
📝 Note: Resonance and exceptions can be challenging, but practice and patience will help you master these concepts.
5. Apply Lewis Dot Structure to Real-World Molecules
To take your Lewis dot structure practice to the next level, apply your skills to real-world molecules. Choose molecules that interest you, such as biomolecules or pharmaceuticals, and construct their Lewis dot structures.
- Example: Construct the Lewis dot structure for glucose (C6H12O6).
- Step 1: Determine the total number of valence electrons (48).
- Step 2: Draw the atoms and represent the electrons as dots.
- Step 3: Recognize the molecular structure and construct the Lewis dot structure.
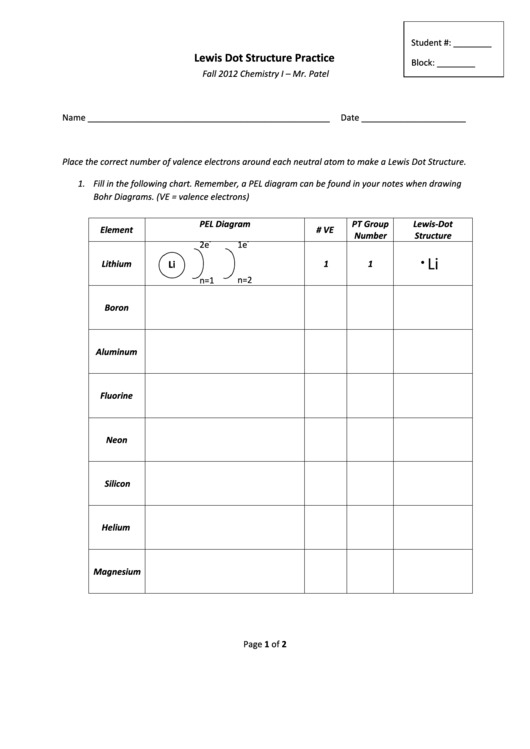
| Molecule | Lewis Dot Structure |
|---|---|
| H2 | H · · · H |
| O2 | O · · · O |
| CO2 | O · · · C · · · O |
By following these five ways to master Lewis dot structure practice, you will become proficient in constructing and understanding the Lewis dot structures of various molecules.
Mastering Lewis dot structure practice takes time and dedication, but with persistence and the right resources, you can excel in chemistry. Remember to practice regularly, focus on resonance and exceptions, and apply your skills to real-world molecules. With these tips, you will become a master of Lewis dot structure practice.
What is the purpose of Lewis dot structure?
+The purpose of Lewis dot structure is to represent the valence electrons of atoms within a molecule, allowing chemists to visualize and understand the bonding and structure of molecules.
How do I construct a Lewis dot structure?
+To construct a Lewis dot structure, determine the total number of valence electrons, draw the atoms and represent the electrons as dots, and connect the atoms with lines, representing shared pairs of electrons.
What are resonance and exceptions in Lewis dot structure?
+Resonance occurs when a molecule has multiple Lewis dot structures that contribute to its overall structure. Exceptions refer to molecules that do not follow the usual rules for constructing Lewis dot structures.