Lewis Dot Diagrams Made Easy: Worksheet Answers Key
Understanding Lewis Dot Diagrams
Lewis dot diagrams, also known as electron dot diagrams, are a simple way to represent the valence electrons of atoms in a molecule. They are a crucial tool for understanding the bonding and structure of molecules. In this post, we will explain the basics of Lewis dot diagrams and provide a worksheet with answers to help you practice.
What is a Lewis Dot Diagram?
A Lewis dot diagram is a graphical representation of the valence electrons of an atom. It is a simple drawing that shows the number of electrons in an atom’s outermost energy level, which is also known as the valence shell. The valence electrons are represented by dots, and the diagram shows how these electrons are arranged around the nucleus of the atom.
How to Draw a Lewis Dot Diagram
Drawing a Lewis dot diagram is a straightforward process. Here are the steps to follow:
- Determine the number of valence electrons in the atom. This can be done by looking at the periodic table and finding the group number of the element.
- Draw the symbol of the atom in the center of the diagram.
- Arrange the valence electrons around the symbol, with each electron represented by a dot.
- The electrons should be arranged in pairs, with each pair separated by a space.
- If there are an odd number of electrons, one pair will have three electrons.
📝 Note: The number of valence electrons in an atom is equal to the group number of the element in the periodic table.
Types of Lewis Dot Diagrams
There are several types of Lewis dot diagrams, including:
- Monatomic ions: These are Lewis dot diagrams of single atoms that have gained or lost electrons to form ions.
- Molecular compounds: These are Lewis dot diagrams of molecules that are composed of two or more atoms.
- Polyatomic ions: These are Lewis dot diagrams of ions that are composed of two or more atoms.
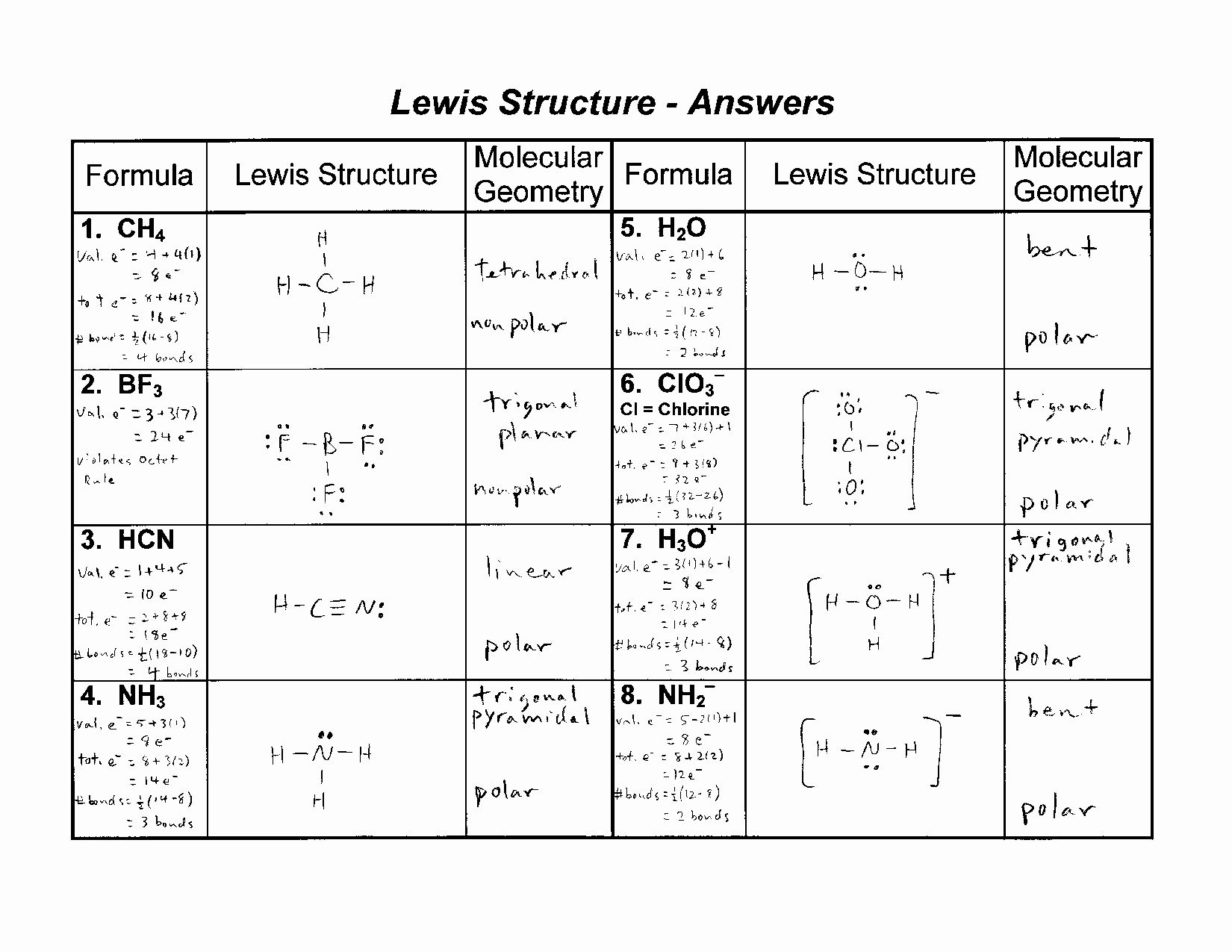
Worksheet Answers Key
Here is a worksheet with 10 questions to help you practice drawing Lewis dot diagrams. The answers are provided below.
Worksheet
- Draw the Lewis dot diagram for the atom with the symbol “C”.
- Draw the Lewis dot diagram for the molecule with the formula “H2O”.
- Draw the Lewis dot diagram for the ion with the symbol “Na+”.
- Draw the Lewis dot diagram for the molecule with the formula “CO2”.
- Draw the Lewis dot diagram for the atom with the symbol “O”.
- Draw the Lewis dot diagram for the molecule with the formula “NH3”.
- Draw the Lewis dot diagram for the ion with the symbol “Cl-”.
- Draw the Lewis dot diagram for the molecule with the formula “CH4”.
- Draw the Lewis dot diagram for the atom with the symbol “N”.
- Draw the Lewis dot diagram for the molecule with the formula “SO2”.
Answers
- · · · · · ·
- H · · · O · · · H
- Na+
- O · · · C · · · O
- · · · · · ·
- H · · · N · · · H
- Cl-
- H · · · C · · · H
- · · · · · ·
- O · · · S · · · O
Common Mistakes to Avoid
When drawing Lewis dot diagrams, there are several common mistakes to avoid:
- Forgetting to include all valence electrons: Make sure to include all valence electrons in the diagram.
- Not arranging electrons in pairs: Electrons should be arranged in pairs, with each pair separated by a space.
- Not using the correct symbol: Use the correct symbol for the atom or molecule being represented.
📝 Note: Lewis dot diagrams are a simple way to represent the valence electrons of atoms in a molecule, but they do not show the actual shape of the molecule.
In summary, Lewis dot diagrams are a useful tool for understanding the bonding and structure of molecules. By following the steps outlined above and practicing with the worksheet, you can become proficient in drawing Lewis dot diagrams.
What is the purpose of a Lewis dot diagram?
+A Lewis dot diagram is a graphical representation of the valence electrons of an atom, used to understand the bonding and structure of molecules.
How many valence electrons does an oxygen atom have?
+An oxygen atom has 6 valence electrons.
What is the difference between a monatomic ion and a polyatomic ion?
+A monatomic ion is a single atom that has gained or lost electrons to form an ion, while a polyatomic ion is an ion composed of two or more atoms.