Lewis Dot Diagram Worksheet Answers Guide
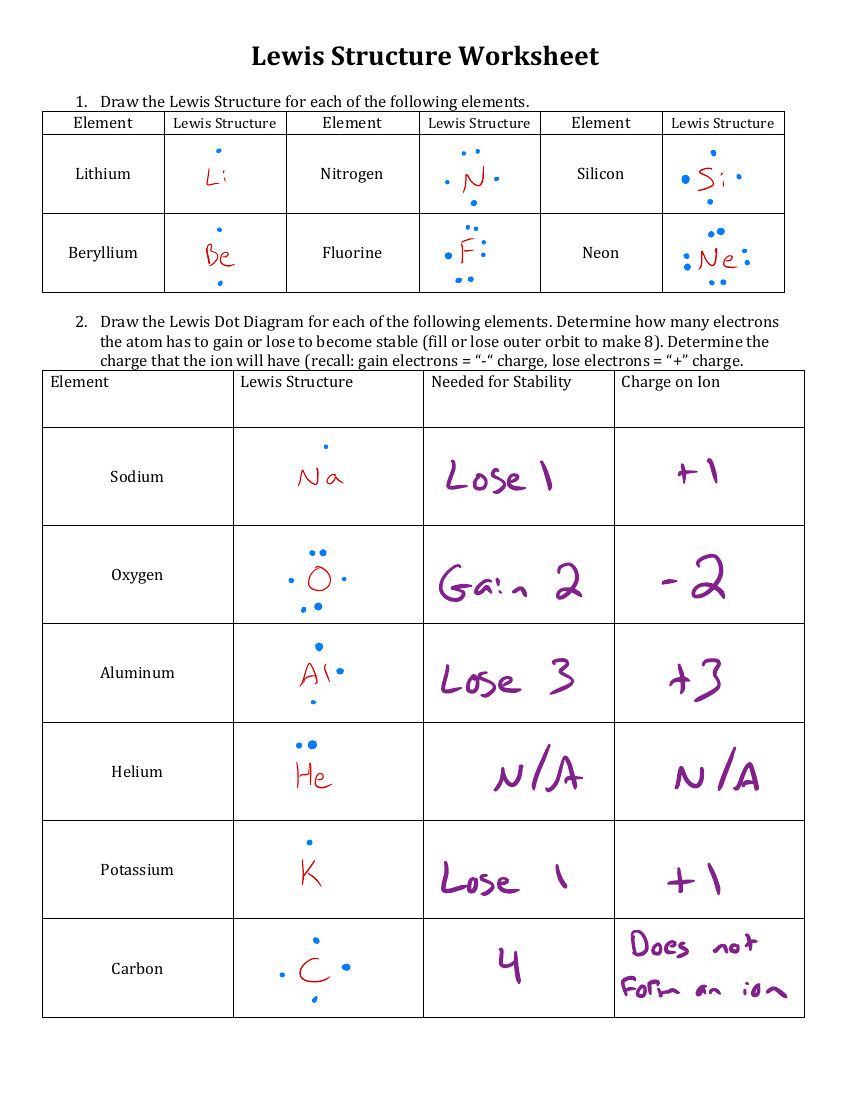
Understanding Lewis Dot Diagrams: A Comprehensive Guide
Lewis dot diagrams are a fundamental concept in chemistry, used to represent the bonding between atoms in a molecule. These diagrams help us visualize the arrangement of electrons in a molecule, which is crucial for understanding chemical properties and behavior. In this guide, we will walk you through the basics of Lewis dot diagrams, provide a step-by-step tutorial on how to create them, and offer a worksheet with answers to help you practice.
What is a Lewis Dot Diagram?
A Lewis dot diagram is a two-dimensional representation of a molecule that uses dots to represent valence electrons. These diagrams are named after Gilbert Lewis, who developed this notation system in the early 20th century. The diagram shows the arrangement of electrons in a molecule, including the number of valence electrons, the type of bonds, and the molecular geometry.
Why are Lewis Dot Diagrams Important?
Lewis dot diagrams are essential for understanding various chemical concepts, including:
- Molecular structure: Lewis dot diagrams help us understand the arrangement of atoms and electrons in a molecule.
- Bonding: These diagrams show the type of bonds between atoms, including single, double, and triple bonds.
- Molecular geometry: Lewis dot diagrams help us predict the shape of a molecule.
- Reactivity: The arrangement of electrons in a molecule can affect its reactivity.
How to Create a Lewis Dot Diagram
Creating a Lewis dot diagram involves a few steps:
- Determine the total number of valence electrons: Calculate the total number of valence electrons in the molecule by adding the valence electrons of each atom.
- Draw the skeleton structure: Draw the basic structure of the molecule, including the arrangement of atoms.
- Add electrons to the atoms: Add valence electrons to each atom, starting with the atom that has the lowest electronegativity.
- Form bonds: Form bonds between atoms by sharing pairs of electrons.
- Check the octet rule: Ensure that each atom has a full outer energy level (eight electrons) by adding lone pairs or adjusting the bonds.
Worksheet: Lewis Dot Diagrams
Practice creating Lewis dot diagrams with the following molecules:

| Molecule | Number of Valence Electrons |
|---|---|
| CH4 | 8 |
| CO2 | 16 |
| H2O | 8 |
| NH3 | 8 |
Answers:
- CH4:
C H H H : :H :H :H - CO2:
O C O :: ::C :: - H2O:
H O H ::H ::O ::H - NH3:
N H H H ::: ::H ::H ::H
👍 Note: Make sure to check the octet rule for each atom and adjust the bonds and lone pairs accordingly.
Common Mistakes to Avoid
When creating Lewis dot diagrams, be aware of the following common mistakes:
- Incorrect number of valence electrons: Double-check the total number of valence electrons to ensure accuracy.
- Incorrect bond formation: Make sure to form bonds between atoms that are adjacent to each other.
- Ignoring the octet rule: Ensure that each atom has a full outer energy level by adding lone pairs or adjusting the bonds.
By following these guidelines and practicing with the worksheet, you’ll become proficient in creating Lewis dot diagrams and understanding the underlying chemical concepts.
In summary, Lewis dot diagrams are a powerful tool for visualizing the arrangement of electrons in a molecule. By mastering this concept, you’ll gain a deeper understanding of molecular structure, bonding, and reactivity. Practice regularly and apply these skills to solve complex chemical problems.
What is the purpose of Lewis dot diagrams?
+Lewis dot diagrams help us understand the arrangement of electrons in a molecule, including the type of bonds and molecular geometry.
How do I determine the total number of valence electrons?
+Calculate the total number of valence electrons by adding the valence electrons of each atom in the molecule.
What is the octet rule?
+The octet rule states that each atom should have a full outer energy level (eight electrons) to achieve stability.