Isotopes Worksheet Answer Key for Students
Understanding Isotopes: A Comprehensive Guide for Students
Isotopes are atoms of the same chemical element that have the same number of protons but differ in the number of neutrons in their atomic nuclei. This concept is crucial in chemistry and physics, and understanding isotopes is essential for students pursuing careers in these fields. In this article, we will delve into the world of isotopes, exploring what they are, how they are formed, and their applications.
What are Isotopes?
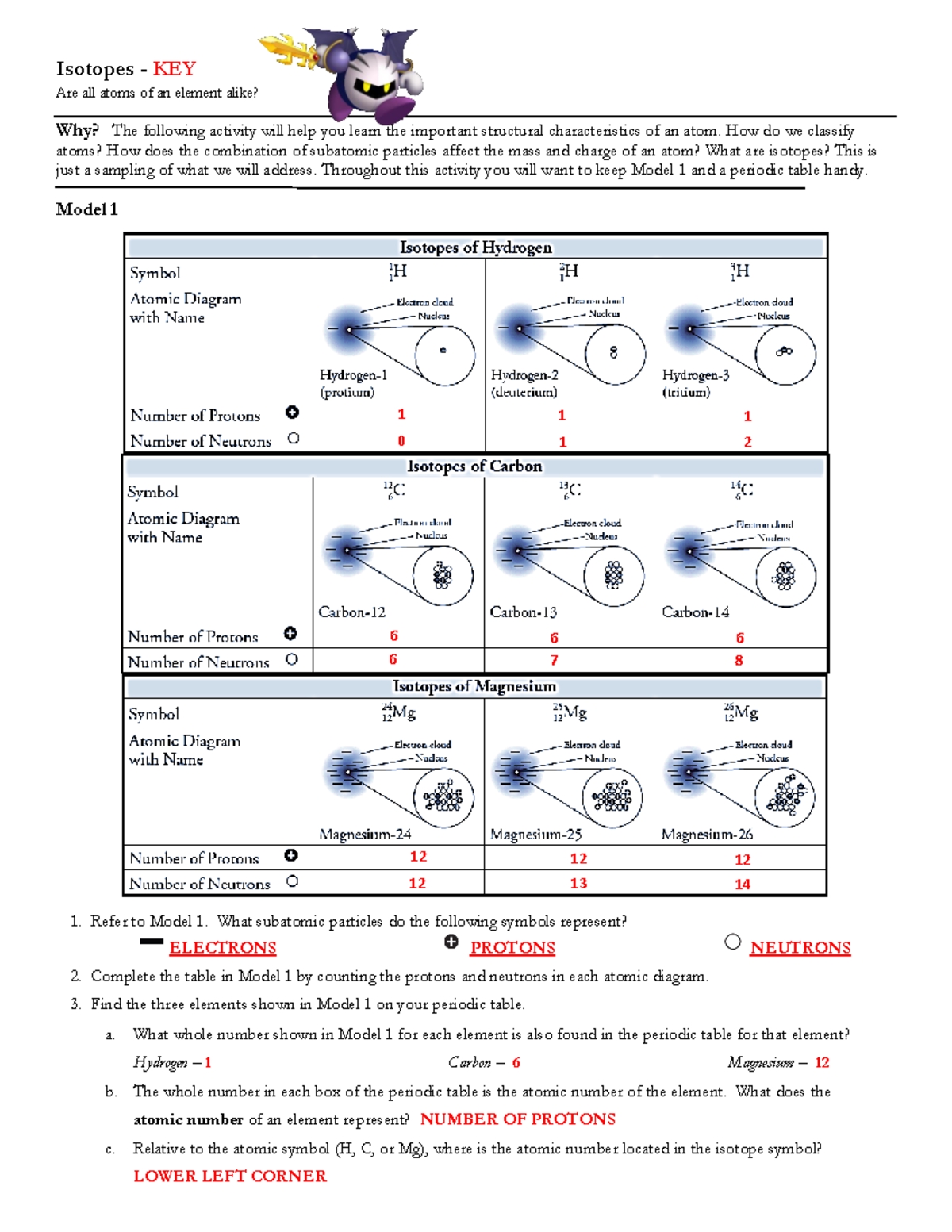
Isotopes are variants of a particular chemical element that share the same number of protons (atomic number) but have different numbers of neutrons. This variation in neutron number leads to differences in atomic mass, while the chemical properties of the element remain largely unchanged. For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon, with 6 protons and 6, 7, and 8 neutrons, respectively.
Types of Isotopes
Isotopes can be classified into two main categories: stable and radioactive isotopes.
- Stable Isotopes: These isotopes do not undergo radioactive decay and remain unchanged over time. Examples include carbon-12 and oxygen-16.
- Radioactive Isotopes: These isotopes are unstable and undergo radioactive decay, emitting radiation as they transform into more stable forms. Examples include carbon-14 and uranium-238.
Formation of Isotopes
Isotopes are formed through various natural and artificial processes, including:
- Nuclear Reactions: Isotopes can be created through nuclear reactions, such as nuclear fission or fusion, which involve the splitting or combining of atomic nuclei.
- Neutron Activation: Exposure to neutron radiation can cause stable isotopes to become radioactive, forming new isotopes through neutron activation.
- Cosmic Ray Interactions: High-energy particles from cosmic rays can interact with atomic nuclei, producing new isotopes through spallation reactions.
Applications of Isotopes
Isotopes have numerous applications in various fields, including:
- Medicine: Radioisotopes are used in medical imaging, cancer treatment, and research, while stable isotopes are used in medical research and diagnostic testing.
- Archaeology: Radiocarbon dating uses the decay of carbon-14 to determine the age of organic materials.
- Environmental Science: Isotopes are used to study climate change, track water movement, and monitor environmental pollution.
- Food and Agriculture: Isotopes are used to study plant growth, track nutrient uptake, and detect food adulteration.
Isotopes Worksheet Answer Key
Here are the answers to a sample isotopes worksheet:
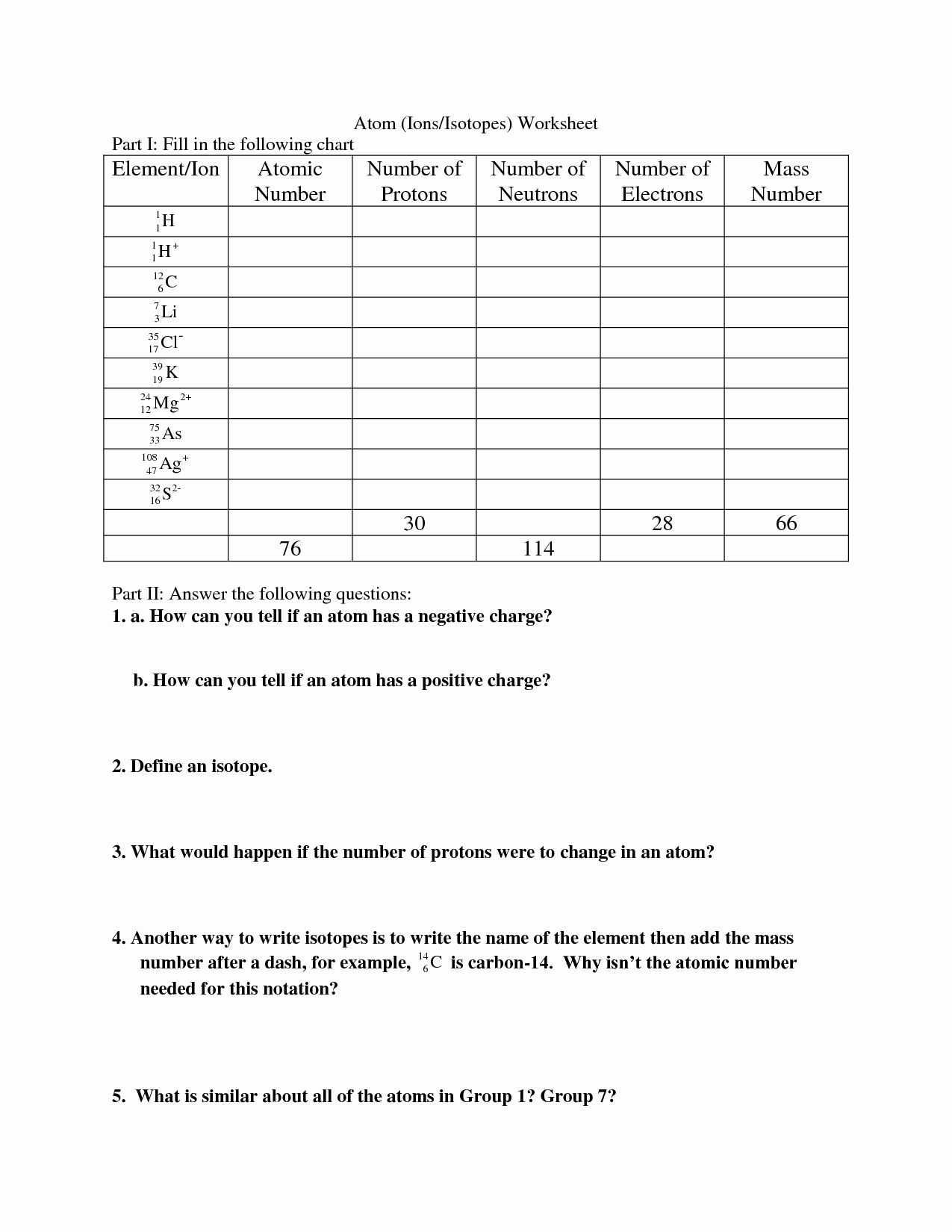
| Question | Answer |
|---|---|
| 1. What is the definition of an isotope? | Atoms of the same chemical element that have the same number of protons but differ in the number of neutrons. |
| 2. Which type of isotope is stable and does not undergo radioactive decay? | Stable isotope |
| 3. What is the process by which a stable isotope becomes radioactive? | Neutron activation |
| 4. What is the application of radiocarbon dating in archaeology? | To determine the age of organic materials |
| 5. Which isotope is used to study plant growth and track nutrient uptake in agriculture? | Carbon-13 |
📝 Note: This worksheet answer key is for sample purposes only and may not reflect actual questions or answers.
In conclusion, isotopes are an essential concept in chemistry and physics, with numerous applications in various fields. Understanding isotopes is crucial for students pursuing careers in these fields, and this article has provided a comprehensive guide to isotopes, including their definition, types, formation, and applications.
What is the difference between a stable and radioactive isotope?
+A stable isotope does not undergo radioactive decay, while a radioactive isotope is unstable and undergoes radioactive decay, emitting radiation.
How are isotopes used in medicine?
+Radioisotopes are used in medical imaging, cancer treatment, and research, while stable isotopes are used in medical research and diagnostic testing.
What is the application of isotopes in environmental science?
+Isotopes are used to study climate change, track water movement, and monitor environmental pollution.