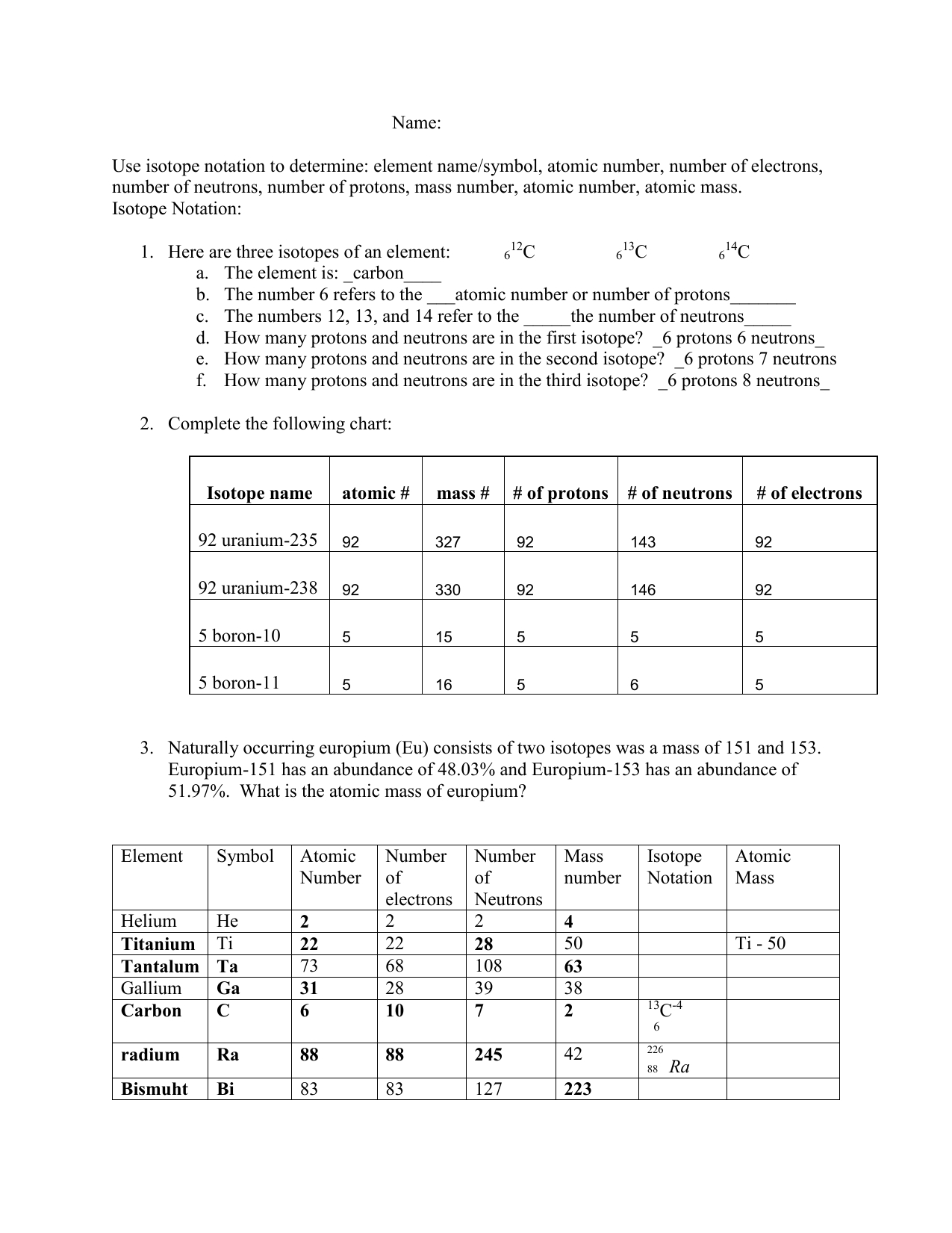
7 Ways to Master Isotope Practice
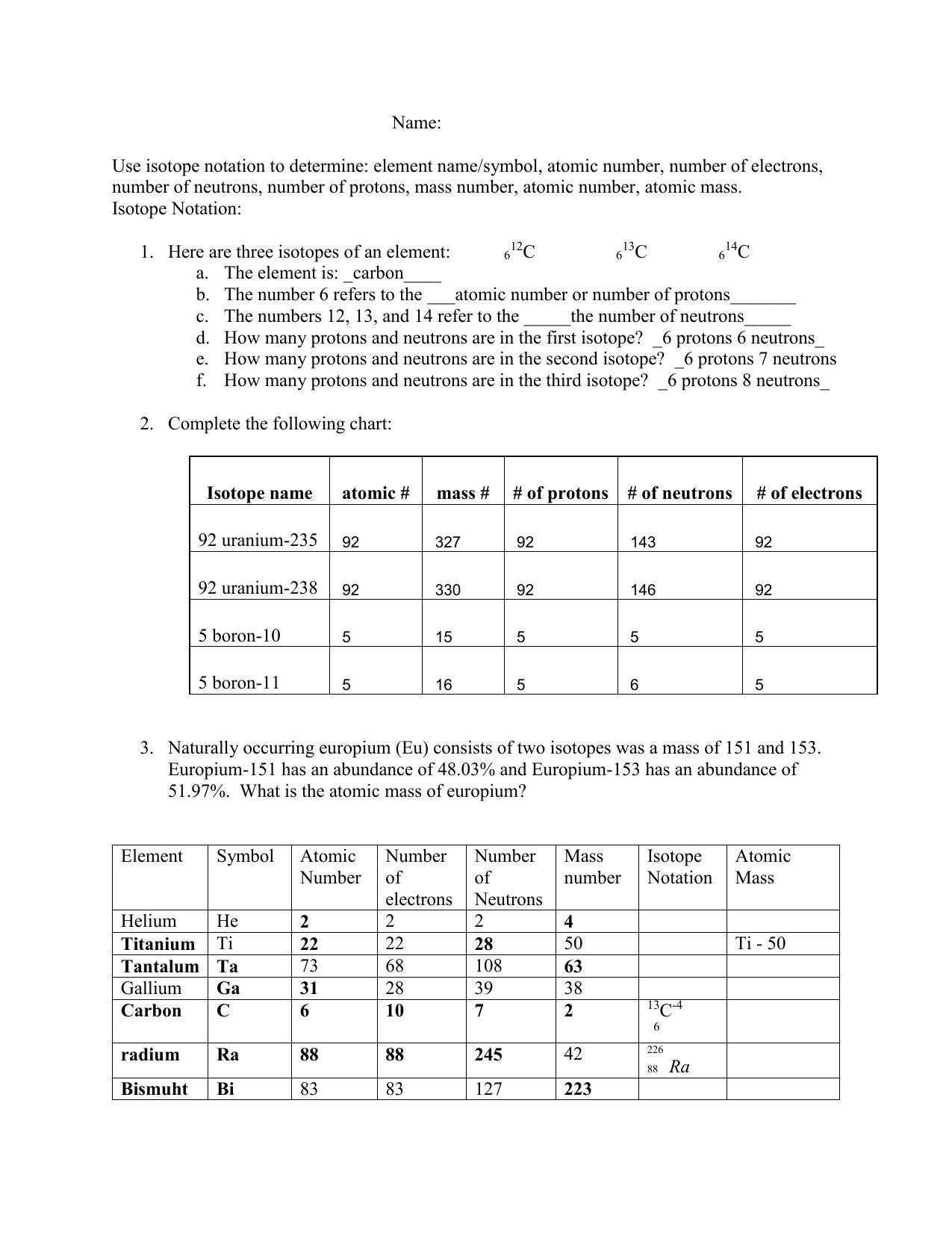
Understanding Isotopes and Their Applications
Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons in their atomic nuclei. This variation in neutron number affects the physical and chemical properties of the isotopes, making them useful in various fields such as medicine, archaeology, and environmental science. Mastering isotope practice requires a deep understanding of the principles and applications of isotopes.
1. Familiarize Yourself with Isotope Fundamentals
To master isotope practice, it’s essential to start with the basics. Here are some key concepts to grasp:
- Isotope notation: Learn how to write isotope notation, which includes the element symbol, mass number (protons + neutrons), and atomic number (protons).
- Isotope types: Understand the different types of isotopes, including stable, radioactive, and cosmogenic isotopes.
- Isotope properties: Familiarize yourself with the physical and chemical properties of isotopes, such as half-life, decay modes, and isotopic abundance.
Isotope Notation Example
For example, the isotope notation for carbon-14 is:
14C
Where:
- C is the element symbol
- 14 is the mass number (6 protons + 8 neutrons)
- 6 is the atomic number (number of protons)
📝 Note: Isotope notation is essential for identifying and communicating about specific isotopes.
2. Explore Isotope Applications in Various Fields
Isotopes have numerous applications across various fields, including:
- Medicine: Isotopes are used in medical imaging, cancer treatment, and disease diagnosis.
- Archaeology: Isotopes are used to date artifacts and reconstruct ancient environments.
- Environmental science: Isotopes are used to study climate change, track water cycles, and monitor pollution.
Isotope Applications in Medicine
For example, positron emission tomography (PET) scans use isotopes like oxygen-15 and nitrogen-13 to diagnose and treat diseases such as cancer and Alzheimer’s.
3. Learn Isotope Measurement Techniques
To work with isotopes, you need to understand various measurement techniques, including:
- Mass spectrometry: Measures the mass-to-charge ratio of ions to identify and quantify isotopes.
- Radiometric dating: Measures the decay rate of radioactive isotopes to determine the age of samples.
- Isotopic analysis: Measures the isotopic composition of samples to reconstruct environmental and geological histories.
Mass Spectrometry Example
For example, a mass spectrometer can measure the mass-to-charge ratio of ions to identify and quantify the isotopes of carbon, such as 12C and 13C.
🔬 Note: Isotope measurement techniques require specialized equipment and expertise.
4. Practice Isotope Calculations and Modeling
To master isotope practice, you need to practice calculations and modeling, including:
- Isotope fractionation: Calculates the difference in isotopic composition between two substances.
- Isotope mixing: Models the mixing of isotopes between different sources.
- Isotope transport: Simulates the transport of isotopes through the environment.
Isotope Fractionation Example
For example, you can calculate the isotope fractionation between two water samples to determine the source of the water.
| Sample | δ18O (‰) |
|---|---|
| Sample A | -10 |
| Sample B | -5 |
📊 Note: Isotope calculations and modeling require a strong understanding of mathematical concepts and isotope principles.
5. Develop Laboratory Skills
Working with isotopes requires hands-on laboratory experience, including:
- Sample preparation: Prepares samples for isotope analysis, such as extracting water or organic matter.
- Instrument operation: Operates mass spectrometers, gas chromatographs, and other equipment used in isotope analysis.
- Data interpretation: Interprets data from isotope measurements, including identifying and quantifying isotopes.
Laboratory Safety
For example, when working with radioactive isotopes, it’s essential to follow proper laboratory safety protocols, such as wearing protective gear and handling samples carefully.
⚠️ Note: Laboratory work with isotopes requires attention to safety protocols and proper training.
6. Stay Up-to-Date with Isotope Research and Developments
The field of isotope science is constantly evolving, with new techniques and applications emerging regularly. To stay current, follow scientific journals, attend conferences, and participate in online forums.
Isotope Research Examples
For example, researchers have recently developed new techniques for measuring isotopes in small samples, such as laser ablation mass spectrometry.
📰 Note: Staying current with isotope research and developments requires ongoing education and engagement with the scientific community.
7. Collaborate with Interdisciplinary Teams
Isotope science is an interdisciplinary field that requires collaboration with experts from various fields, including geology, biology, chemistry, and physics. Working with interdisciplinary teams can help you tackle complex problems and develop innovative solutions.
Interdisciplinary Collaboration Example
For example, a team of researchers from geology, biology, and chemistry collaborated to study the isotopic composition of ancient plants and reconstruct past climates.
🤝 Note: Interdisciplinary collaboration requires effective communication, respect for different disciplines, and a willingness to learn from others.
By following these seven steps, you can master isotope practice and develop a deep understanding of the principles and applications of isotopes.
In summary, mastering isotope practice requires a combination of theoretical knowledge, laboratory skills, and interdisciplinary collaboration. By following these steps, you can develop a strong foundation in isotope science and stay up-to-date with the latest research and developments.
What is the difference between a stable and radioactive isotope?
+A stable isotope has a stable nucleus and does not undergo radioactive decay, whereas a radioactive isotope has an unstable nucleus and undergoes radioactive decay.
What is the purpose of isotopic analysis?
+Isotopic analysis is used to reconstruct environmental and geological histories, track the movement of substances through the environment, and identify the sources of substances.
What is the difference between mass spectrometry and radiometric dating?
+Mass spectrometry measures the mass-to-charge ratio of ions to identify and quantify isotopes, whereas radiometric dating measures the decay rate of radioactive isotopes to determine the age of samples.