5 Tips to Master Isotope Notation in Chemistry
Understanding Isotope Notation in Chemistry
Isotope notation is a fundamental concept in chemistry that allows scientists to identify and distinguish between different isotopes of an element. Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. Mastering isotope notation is crucial for chemists, physicists, and students of chemistry to accurately communicate and understand the properties and behaviors of elements. In this article, we will explore five tips to help you master isotope notation in chemistry.
Tips to Master Isotope Notation
1. Understand the Basics of Isotope Notation
Isotope notation is written in the format:
AZX
Where:
- X is the symbol of the element
- Z is the atomic number (number of protons in the nucleus)
- A is the mass number (sum of protons and neutrons in the nucleus)
For example, the isotope notation for carbon-12 is:
126C
This notation tells us that the element is carbon (X = C), it has an atomic number of 6 (Z = 6), and a mass number of 12 (A = 12).
📝 Note: The mass number (A) is not always written, but it is essential to understand its significance in isotope notation.
2. Learn the Rules for Writing Isotope Notation
There are specific rules to follow when writing isotope notation:
- The atomic number (Z) is always written as a subscript to the left of the element symbol.
- The mass number (A) is written as a superscript to the left of the element symbol.
- If the mass number is not written, it is assumed to be the most stable or naturally occurring isotope.
For example:
- 126C is the correct notation for carbon-12.
- 6C is the correct notation for carbon with an atomic number of 6, but without specifying the mass number.
3. Practice, Practice, Practice
Practice is key to mastering isotope notation. Try writing the notation for different elements and isotopes, and check your answers with a reference source.
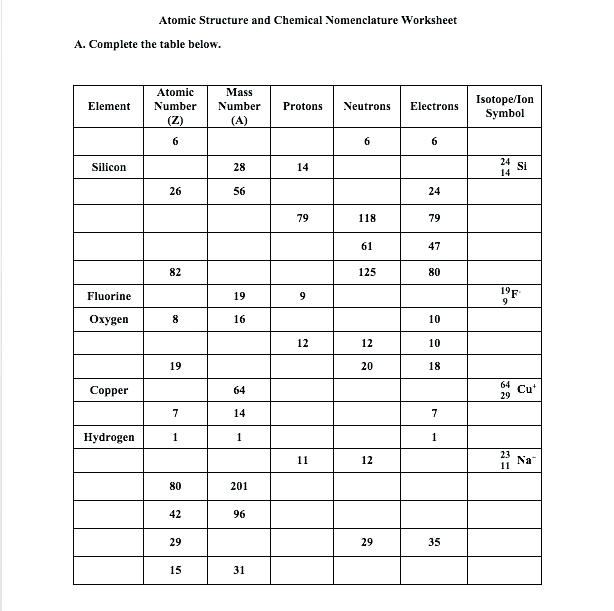
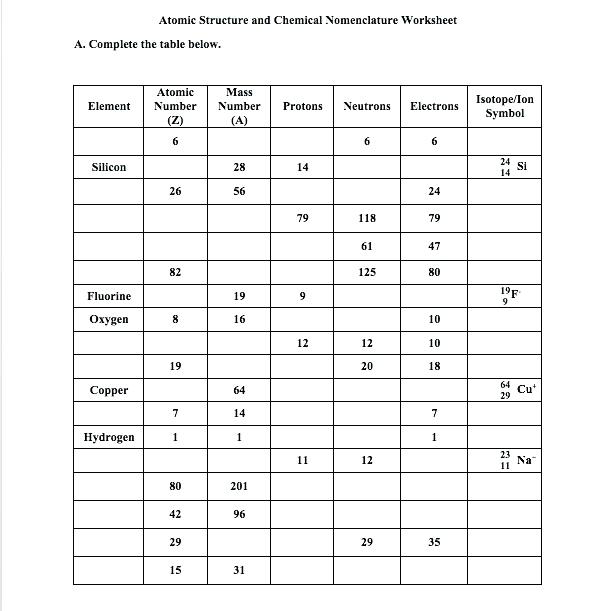
| Element | Atomic Number | Mass Number | Isotope Notation |
|---|---|---|---|
| Hydrogen | 1 | 1 | 11H |
| Carbon | 6 | 12 | 126C |
| Oxygen | 8 | 16 | 168O |
4. Understand the Significance of Isotope Notation
Isotope notation is essential in chemistry because it allows scientists to:
- Identify specific isotopes of an element
- Determine the number of neutrons in an isotope
- Calculate the atomic mass of an element
- Understand the properties and behaviors of elements
For example, the isotope notation for carbon-14 (146C) tells us that this isotope has two more neutrons than carbon-12 (126C). This information is crucial in understanding the properties and behaviors of these isotopes.
5. Learn to Identify Common Isotopes
Some isotopes are more common than others, and it’s essential to be familiar with these. For example:
- Carbon-12 (126C) is the most stable and naturally occurring isotope of carbon.
- Oxygen-16 (168O) is the most stable and naturally occurring isotope of oxygen.
By mastering isotope notation, you’ll be able to identify and distinguish between different isotopes of an element, and understand their properties and behaviors.
Summarizing the key points, mastering isotope notation in chemistry requires understanding the basics, learning the rules, practicing, understanding the significance, and identifying common isotopes. By following these tips, you’ll become proficient in writing and interpreting isotope notation, which is essential for success in chemistry.
What is the purpose of isotope notation in chemistry?
+
Isotope notation is used to identify and distinguish between different isotopes of an element, allowing scientists to understand their properties and behaviors.
What is the correct format for writing isotope notation?
+
The correct format for writing isotope notation is AZX, where X is the symbol of the element, Z is the atomic number, and A is the mass number.
Why is it essential to understand isotope notation in chemistry?
+
Understanding isotope notation is crucial in chemistry because it allows scientists to identify specific isotopes, determine the number of neutrons, calculate atomic mass, and understand the properties and behaviors of elements.
Related Terms:
- Isotope notation Worksheet answers
- Isotope Practice Worksheet answers PDF
- Isotope Practice problems Worksheet
- Chemistry Worksheet isotope notation
- Isotope notation examples
- Nuclear notation Worksheet answers