Periodic Table Worksheet for Students

Unlocking the Secrets of the Periodic Table: A Comprehensive Worksheet for Students
The periodic table is a powerful tool used in chemistry to organize elements based on their properties and electron configurations. As a student, understanding the periodic table is crucial for success in chemistry and other science-related fields. In this worksheet, we will delve into the world of the periodic table, exploring its structure, components, and significance.
Understanding the Structure of the Periodic Table
The periodic table is arranged in a logical and systematic way, with elements listed in order of increasing atomic number (number of protons in the nucleus). The table is divided into rows called periods and columns called groups or families.
- Periods: Elements in the same period have the same number of electron shells. As you move from left to right across a period, the number of electrons in the outermost energy level increases.
- Groups: Elements in the same group have similar chemical properties due to the same number of electrons in their outermost energy level.
Components of the Periodic Table
The periodic table consists of several key components:
- Elements: The building blocks of matter, elements are represented by symbols (e.g., H for hydrogen) and have unique properties.
- Atomic Number: The number of protons in an element’s nucleus, which determines its position in the periodic table.
- Atomic Mass: The total number of protons and neutrons in an element’s nucleus, which affects its physical properties.
- Electron Configuration: The arrangement of electrons in an atom, which influences an element’s chemical properties.
Identifying Elements on the Periodic Table
To identify an element on the periodic table, you need to know its symbol, atomic number, and atomic mass. Here’s a step-by-step guide:
- Locate the element’s symbol on the periodic table.
- Find the element’s atomic number, which is usually listed above or below the symbol.
- Determine the element’s atomic mass, which is often listed below the symbol.
🔍 Note: Some periodic tables may not display the atomic mass. In this case, you can use an online periodic table or a reference book to find the information.
Understanding Families of Elements
Elements in the same group or family exhibit similar chemical properties due to the same number of electrons in their outermost energy level. Some notable families include:
- Alkali Metals (Group 1): Highly reactive elements that readily lose one electron to form a positive ion.
- Noble Gases (Group 18): Unreactive elements that have a full outer energy level and do not readily react with other elements.
- Halogens (Group 17): Highly reactive elements that readily gain one electron to form a negative ion.
Periodic Trends
As you move across a period or down a group, you’ll notice trends in various properties, such as:
- Atomic Radius: Decreases across a period, increases down a group
- Electronegativity: Increases across a period, decreases down a group
- Ionization Energy: Increases across a period, decreases down a group
📊 Note: Understanding periodic trends can help you predict an element's properties and behavior.
Applying the Periodic Table in Real-Life Scenarios
The periodic table has numerous practical applications in fields like chemistry, physics, and engineering. Here are a few examples:
- Materials Science: Understanding the properties of elements and their combinations helps develop new materials with specific characteristics.
- Energy Production: Knowledge of the periodic table is crucial for developing nuclear power plants and understanding radiation safety.
- Environmental Science: The periodic table helps scientists understand the behavior of pollutants and develop strategies for environmental remediation.
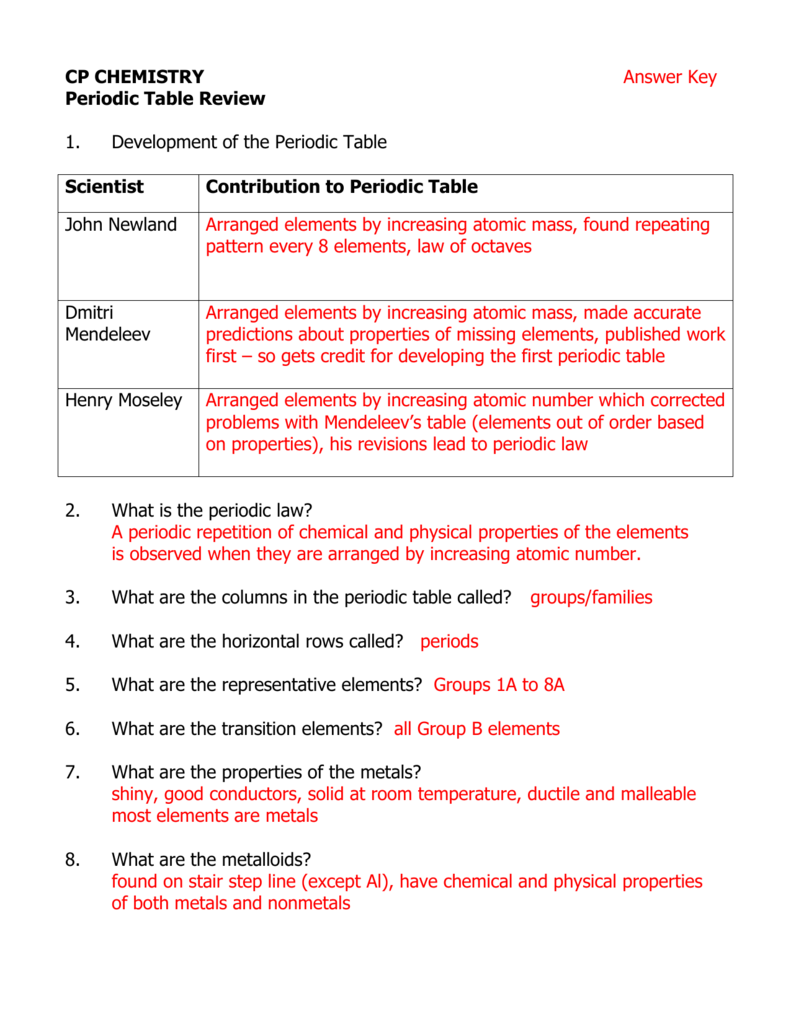
| Element | Symbol | Atomic Number | Atomic Mass |
|---|---|---|---|
| Hydrogen | H | 1 | 1.00794 |
| Helium | He | 2 | 4.002602 |
| Oxygen | O | 8 | 15.9994 |
In conclusion, the periodic table is a powerful tool that helps us understand the properties and behavior of elements. By mastering the structure, components, and trends of the periodic table, you’ll be better equipped to succeed in chemistry and other science-related fields. Remember to practice identifying elements, understanding families, and applying periodic trends to real-life scenarios.
What is the significance of the periodic table in chemistry?
+The periodic table is a crucial tool in chemistry, allowing scientists to organize elements based on their properties and electron configurations. It helps predict an element’s behavior, identify relationships between elements, and understand chemical reactions.
How do I identify an element on the periodic table?
+To identify an element on the periodic table, locate its symbol, atomic number, and atomic mass. You can find this information on the periodic table or in a reference book.
What are some practical applications of the periodic table?
+The periodic table has numerous practical applications in fields like materials science, energy production, and environmental science. It helps scientists develop new materials, understand nuclear reactions, and predict the behavior of pollutants.
Related Terms:
- Periodic table Review Worksheet PDF
- Periodic table families Worksheet PDF
- Periodic trends worksheet doc