Isotope and Ion Practice Worksheet Answer Key
Isotopes and Ions: Understanding the Basics
Isotopes and ions are two fundamental concepts in chemistry that help us understand the structure and properties of atoms. In this article, we will delve into the world of isotopes and ions, exploring their definitions, types, and importance in chemistry.
What are Isotopes?
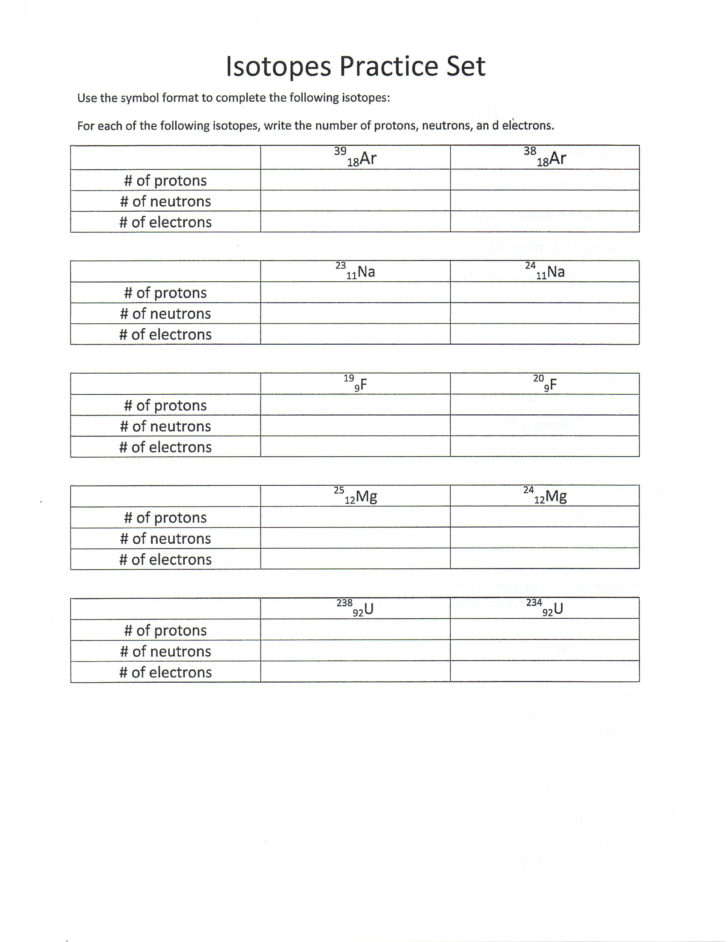
Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This means that isotopes have the same atomic number (number of protons) but different mass numbers (total number of protons and neutrons).
For example, carbon-12, carbon-13, and carbon-14 are all isotopes of the element carbon. They all have 6 protons in their atomic nuclei but differ in the number of neutrons: carbon-12 has 6 neutrons, carbon-13 has 7 neutrons, and carbon-14 has 8 neutrons.
Types of Isotopes
There are two main types of isotopes: stable isotopes and radioactive isotopes.
- Stable Isotopes: These isotopes do not undergo radioactive decay and remain stable over time. Examples of stable isotopes include carbon-12 and oxygen-16.
- Radioactive Isotopes: These isotopes undergo radioactive decay, emitting radiation as they transform into more stable isotopes. Examples of radioactive isotopes include carbon-14 and uranium-238.
What are Ions?
Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. Cations are positively charged ions, while anions are negatively charged ions.
For example, when a sodium atom loses an electron, it becomes a positively charged sodium ion (Na+). Conversely, when a chlorine atom gains an electron, it becomes a negatively charged chloride ion (Cl-).
Types of Ions
There are two main types of ions: monatomic ions and polyatomic ions.
- Monatomic Ions: These ions consist of a single atom that has gained or lost electrons. Examples of monatomic ions include sodium ions (Na+) and chloride ions (Cl-).
- Polyatomic Ions: These ions consist of multiple atoms that have gained or lost electrons. Examples of polyatomic ions include ammonium ions (NH4+) and carbonate ions (CO32-).

| Isotope/Ion | Atomic Number | Mass Number | Charge |
|---|---|---|---|
| Carbon-12 | 6 | 12 | 0 |
| Carbon-14 | 6 | 14 | 0 |
| Sodium Ion (Na+) | 11 | 23 | +1 |
| Chloride Ion (Cl-) | 17 | 35.5 | -1 |
📝 Note: The table above provides examples of isotopes and ions, highlighting their atomic numbers, mass numbers, and charges.
In conclusion, isotopes and ions are essential concepts in chemistry that help us understand the structure and properties of atoms. By recognizing the differences between stable and radioactive isotopes, as well as monatomic and polyatomic ions, we can better comprehend the behavior of elements and their interactions.
What is the main difference between isotopes and ions?
+Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons, while ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge.
What are the two main types of isotopes?
+Stable isotopes and radioactive isotopes. Stable isotopes do not undergo radioactive decay, while radioactive isotopes undergo radioactive decay, emitting radiation as they transform into more stable isotopes.
What is the difference between monatomic and polyatomic ions?
+Monatomic ions consist of a single atom that has gained or lost electrons, while polyatomic ions consist of multiple atoms that have gained or lost electrons.