Ionic Compound Formula Writing Made Easy
Understanding Ionic Compounds
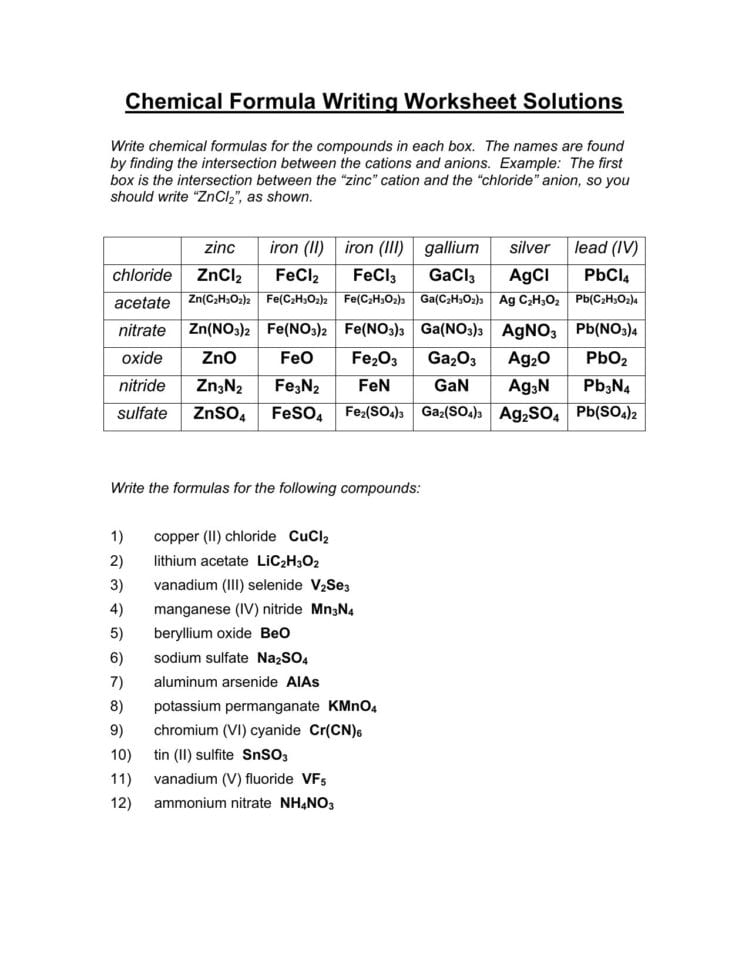
Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. The electrostatic attraction between these ions holds them together, creating a strong chemical bond. To write the formula for an ionic compound, you need to know the symbols of the ions involved, their charges, and the number of ions required to balance the charges.
Identifying Ions and Their Charges
To write the formula for an ionic compound, you need to identify the ions involved and their charges. Cations (positive ions) are typically formed when metal atoms lose one or more electrons, while anions (negative ions) are formed when nonmetal atoms gain one or more electrons.
- Cations: Metal ions have a positive charge, which is equal to the number of electrons lost by the metal atom. For example:
- Sodium (Na) loses one electron to form a sodium ion (Na+) with a +1 charge.
- Calcium (Ca) loses two electrons to form a calcium ion (Ca2+) with a +2 charge.
- Anions: Nonmetal ions have a negative charge, which is equal to the number of electrons gained by the nonmetal atom. For example:
- Chlorine (Cl) gains one electron to form a chloride ion (Cl-) with a -1 charge.
- Oxygen (O) gains two electrons to form an oxide ion (O2-) with a -2 charge.
Writing Ionic Compound Formulas
To write the formula for an ionic compound, follow these steps:
- Write the symbol of the cation first, followed by the symbol of the anion.
- Determine the charges of the ions and balance them to ensure the compound is neutral.
- Use parentheses and subscripts to indicate the number of ions required to balance the charges.
Example 1: Sodium chloride (NaCl)
- Cation: Na+ (sodium ion with a +1 charge)
- Anion: Cl- (chloride ion with a -1 charge)
- Formula: NaCl (one sodium ion and one chloride ion)
Example 2: Calcium oxide (CaO)
- Cation: Ca2+ (calcium ion with a +2 charge)
- Anion: O2- (oxide ion with a -2 charge)
- Formula: CaO (one calcium ion and one oxide ion)
Example 3: Aluminum sulfate (Al2(SO4)3)
- Cation: Al3+ (aluminum ion with a +3 charge)
- Anion: SO42- (sulfate ion with a -2 charge)
- Formula: Al2(SO4)3 (two aluminum ions and three sulfate ions)
💡 Note: When writing ionic compound formulas, make sure to balance the charges of the ions. If the charges are not balanced, the compound will not be neutral, and the formula will be incorrect.
Common Pitfalls to Avoid
When writing ionic compound formulas, avoid the following common pitfalls:
- Incorrect charge balancing: Make sure to balance the charges of the ions to ensure the compound is neutral.
- Incorrect use of parentheses and subscripts: Use parentheses and subscripts correctly to indicate the number of ions required to balance the charges.
- Incorrect order of ions: Write the symbol of the cation first, followed by the symbol of the anion.
Conclusion
Writing ionic compound formulas can be a challenging task, but by following the steps outlined above and avoiding common pitfalls, you can master this skill. Remember to identify the ions involved, determine their charges, and balance them to ensure the compound is neutral. With practice, you will become proficient in writing ionic compound formulas.
What is an ionic compound?
+An ionic compound is a type of chemical compound that is formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges.
How do I write the formula for an ionic compound?
+To write the formula for an ionic compound, identify the ions involved, determine their charges, and balance them to ensure the compound is neutral. Use parentheses and subscripts to indicate the number of ions required to balance the charges.
What are common pitfalls to avoid when writing ionic compound formulas?
+Common pitfalls to avoid include incorrect charge balancing, incorrect use of parentheses and subscripts, and incorrect order of ions.