Understanding Ionic Bonds with a Worksheet
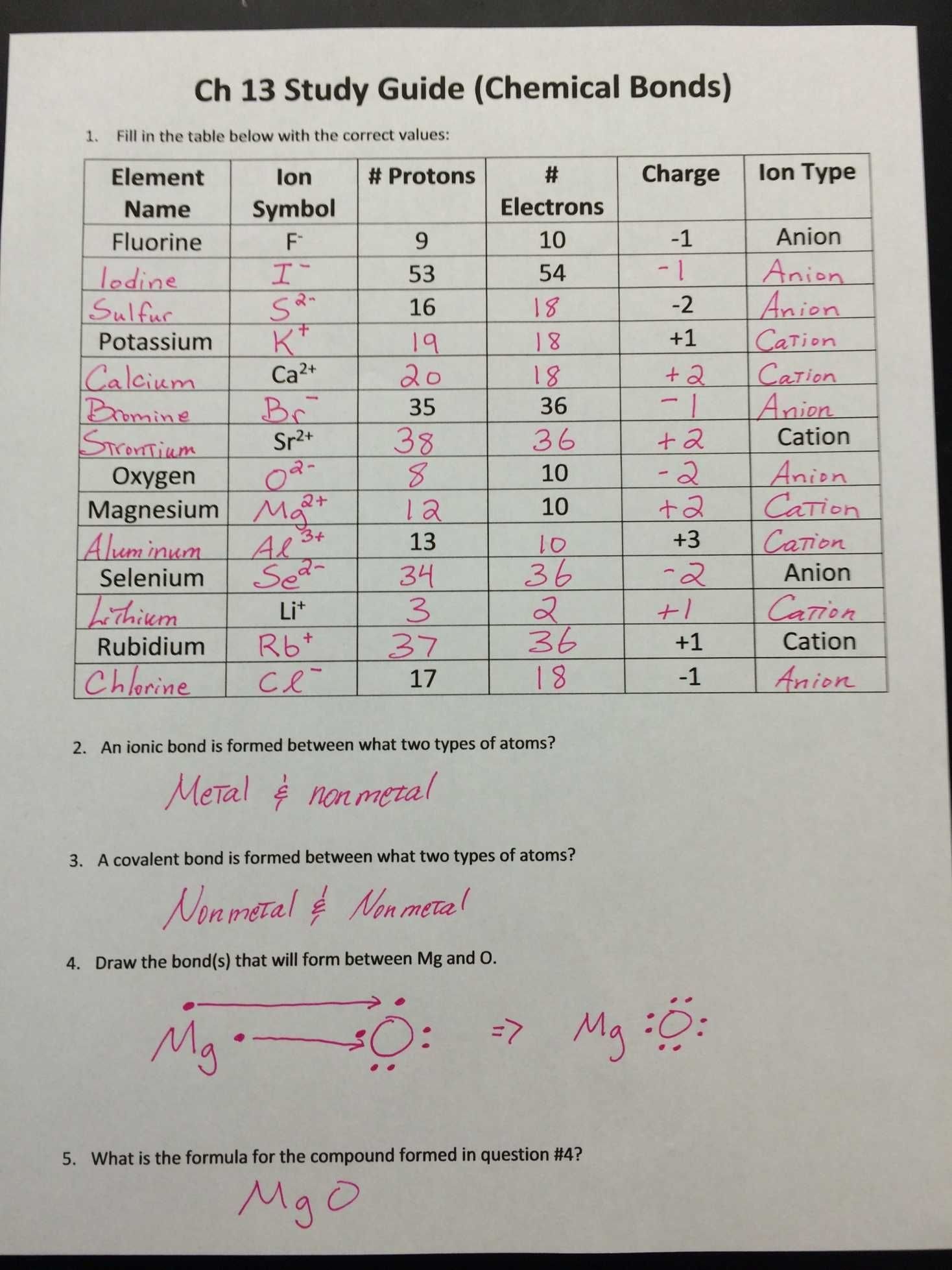
Understanding Ionic Bonds with a Worksheet
Ionic bonds are a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. These ions are then attracted to each other, forming a strong electrostatic bond. In this article, we will explore the concept of ionic bonds in more detail and provide a worksheet to help you better understand this concept.
What are Ionic Bonds?
Ionic bonds are typically formed between metals and nonmetals. Metals tend to lose electrons to form positively charged ions, known as cations, while nonmetals tend to gain electrons to form negatively charged ions, known as anions. The electrostatic attraction between the cations and anions results in the formation of an ionic bond.
For example, when sodium (Na) reacts with chlorine (Cl), the sodium atom loses an electron to form a positively charged ion (Na+), while the chlorine atom gains an electron to form a negatively charged ion (Cl-). The electrostatic attraction between the Na+ and Cl- ions results in the formation of an ionic bond, resulting in the formation of sodium chloride (NaCl), also known as table salt.
How are Ionic Bonds Formed?
Ionic bonds are formed through a process known as electron transfer. This process involves the transfer of electrons from one atom to another, resulting in the formation of ions with opposite charges.
Here are the steps involved in the formation of an ionic bond:
- Step 1: The atoms involved in the reaction come into contact with each other.
- Step 2: One or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
- Step 3: The ions with opposite charges are attracted to each other, resulting in the formation of an ionic bond.
📝 Note: The number of electrons transferred during the formation of an ionic bond depends on the number of electrons required to achieve a stable electronic configuration.
Properties of Ionic Bonds
Ionic bonds have several properties that distinguish them from other types of chemical bonds. Some of the key properties of ionic bonds include:
- High melting and boiling points: Ionic compounds tend to have high melting and boiling points due to the strong electrostatic attraction between the ions.
- High hardness: Ionic compounds tend to be hard and brittle due to the strong electrostatic attraction between the ions.
- Conductivity: Ionic compounds tend to be poor conductors of electricity in solid form, but become good conductors when dissolved in water.
- Solubility: Ionic compounds tend to be soluble in water due to the ability of the ions to interact with water molecules.
Worksheet: Understanding Ionic Bonds
Use the following worksheet to test your understanding of ionic bonds:

| Compound | Cation | Anion | Number of Electrons Transferred |
|---|---|---|---|
| NaCl | Na+ | Cl- | 1 |
| CaO | Ca2+ | O2- | 2 |
| MgCl2 | Mg2+ | Cl- | 2 |
| KBr | K+ | Br- | 1 |
Instructions: Fill in the blanks in the table above to complete the worksheet.
Answers:
| Compound | Cation | Anion | Number of Electrons Transferred |
|---|---|---|---|
| NaCl | Na+ | Cl- | 1 |
| CaO | Ca2+ | O2- | 2 |
| MgCl2 | Mg2+ | Cl- | 2 |
| KBr | K+ | Br- | 1 |
Conclusion
Ionic bonds are a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. These ions are then attracted to each other, forming a strong electrostatic bond. In this article, we explored the concept of ionic bonds in more detail and provided a worksheet to help you better understand this concept.
By completing the worksheet above, you should have a better understanding of how ionic bonds are formed and the properties of ionic compounds.
What is an ionic bond?
+An ionic bond is a type of chemical bond that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges.
How are ionic bonds formed?
+Ionic bonds are formed through a process known as electron transfer, where one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
What are the properties of ionic bonds?
+Ionic bonds have several properties, including high melting and boiling points, high hardness, poor conductivity in solid form, and high solubility in water.