5 Ways to Master Ionic Bonding in Chemistry
Understanding Ionic Bonding: A Fundamental Concept in Chemistry
Ionic bonding is a crucial concept in chemistry that helps us understand how atoms interact with each other to form compounds. It is a type of chemical bonding that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. In this blog post, we will explore five ways to master ionic bonding in chemistry, making it easier for you to grasp this fundamental concept.
1. Learn the Basics of Ionic Bonding
Before diving into the details of ionic bonding, it’s essential to understand the basics. Ionic bonding occurs when one or more electrons are transferred from a metal atom to a non-metal atom. This transfer of electrons results in the formation of ions with opposite charges. The metal atom loses one or more electrons to become a positively charged ion, known as a cation. The non-metal atom gains one or more electrons to become a negatively charged ion, known as an anion.
Key Terms to Remember:
- Cation: A positively charged ion formed when a metal atom loses one or more electrons.
- Anion: A negatively charged ion formed when a non-metal atom gains one or more electrons.
2. Practice Identifying Ionic Compounds
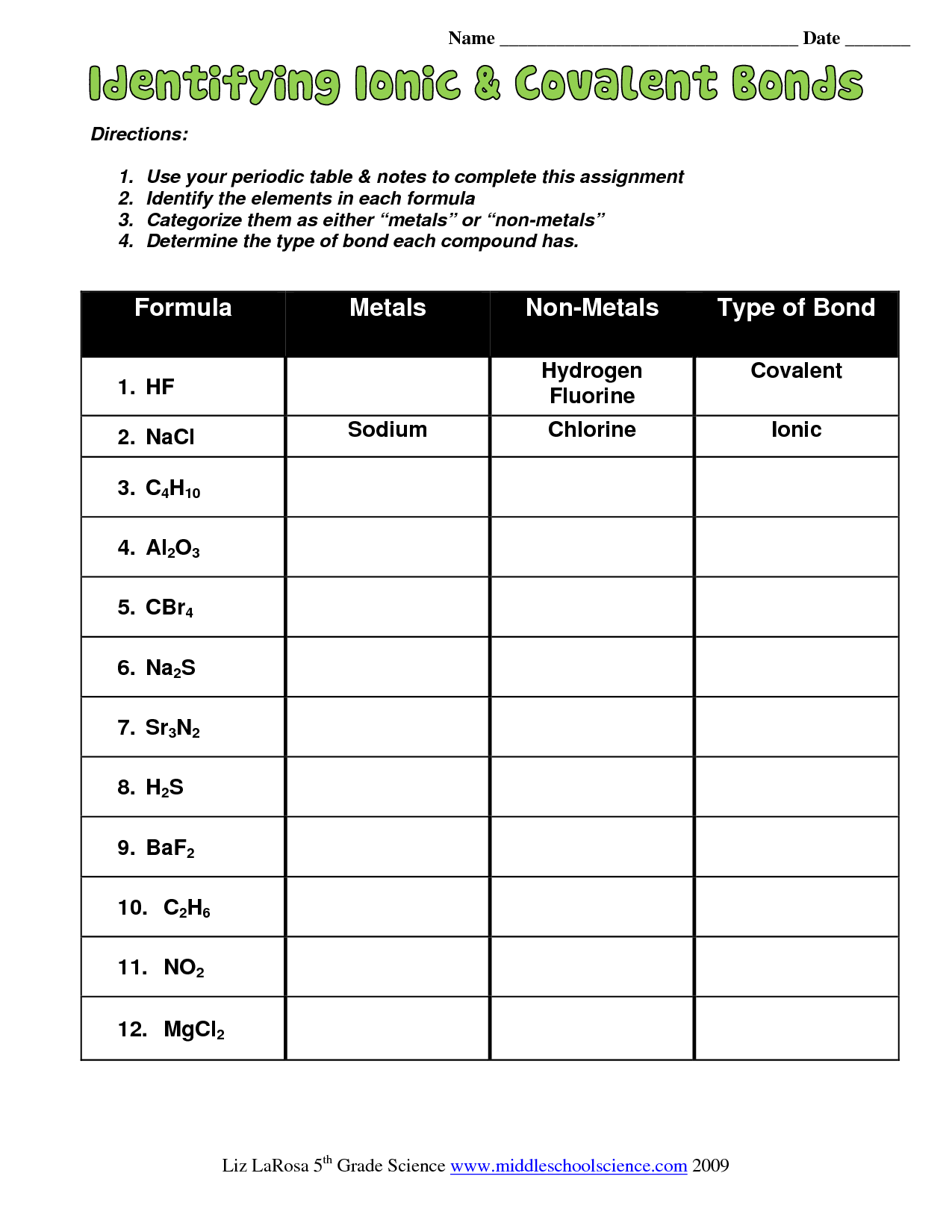
To master ionic bonding, you need to be able to identify ionic compounds. An ionic compound is a compound that consists of ions with opposite charges. Here are some examples of ionic compounds:
- Sodium chloride (NaCl)
- Calcium carbonate (CaCO3)
- Aluminum oxide (Al2O3)
Tips for Identifying Ionic Compounds:
- Look for the presence of a metal atom and a non-metal atom.
- Check if the metal atom has lost one or more electrons to become a cation.
- Check if the non-metal atom has gained one or more electrons to become an anion.
3. Understand the Octet Rule
The octet rule is a fundamental principle in chemistry that states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. In ionic bonding, the octet rule helps us predict the number of electrons that will be transferred between atoms.
How to Apply the Octet Rule:
- Identify the number of valence electrons in the metal and non-metal atoms.
- Determine the number of electrons that need to be transferred to achieve a full outer energy level.
- Use the octet rule to predict the formula of the ionic compound.
4. Visualize Ionic Compounds Using Lewis Structures
Lewis structures are a powerful tool for visualizing the structure of ionic compounds. A Lewis structure shows the arrangement of electrons in a compound, making it easier to understand the bonding between atoms.
How to Draw Lewis Structures:
- Identify the metal and non-metal atoms in the compound.
- Determine the number of valence electrons in each atom.
- Draw the Lewis structure by arranging the electrons in a way that satisfies the octet rule.
5. Practice Writing Formulas for Ionic Compounds
Writing formulas for ionic compounds is an essential skill in chemistry. To write a formula, you need to know the charges of the ions involved and the number of electrons that have been transferred.
Tips for Writing Formulas:
- Identify the charges of the ions involved.
- Determine the number of electrons that have been transferred.
- Use the charges and number of electrons to write the formula of the ionic compound.
📝 Note: Practice is key to mastering ionic bonding. Make sure to practice writing formulas and drawing Lewis structures to reinforce your understanding of ionic bonding.
What is the difference between a cation and an anion?
+A cation is a positively charged ion formed when a metal atom loses one or more electrons. An anion is a negatively charged ion formed when a non-metal atom gains one or more electrons.
How do I identify an ionic compound?
+To identify an ionic compound, look for the presence of a metal atom and a non-metal atom. Check if the metal atom has lost one or more electrons to become a cation and if the non-metal atom has gained one or more electrons to become an anion.
What is the octet rule?
+The octet rule is a fundamental principle in chemistry that states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons.
In conclusion, mastering ionic bonding in chemistry requires a solid understanding of the basics, practice identifying ionic compounds, understanding the octet rule, visualizing ionic compounds using Lewis structures, and writing formulas for ionic compounds. By following these five ways, you’ll be well on your way to becoming proficient in ionic bonding and unlocking the secrets of chemistry.
Related Terms:
- List of ionic compounds pdf