5 Ways to Master Ionic and Covalent Bonds Coloring

Understanding Ionic and Covalent Bonds: The Foundation of Chemistry
Chemical bonding is a fundamental concept in chemistry, and mastering ionic and covalent bonds is essential for understanding the structure and properties of molecules. Ionic and covalent bonds are the two primary types of chemical bonds that hold atoms together to form molecules. In this article, we will explore the world of ionic and covalent bonds, and provide you with five ways to master their coloring.
What are Ionic Bonds?
Ionic bonds are formed when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between the positively charged cation and the negatively charged anion holds the ions together, forming a strong chemical bond. Ionic bonds are typically found in compounds that consist of metals and nonmetals.
What are Covalent Bonds?
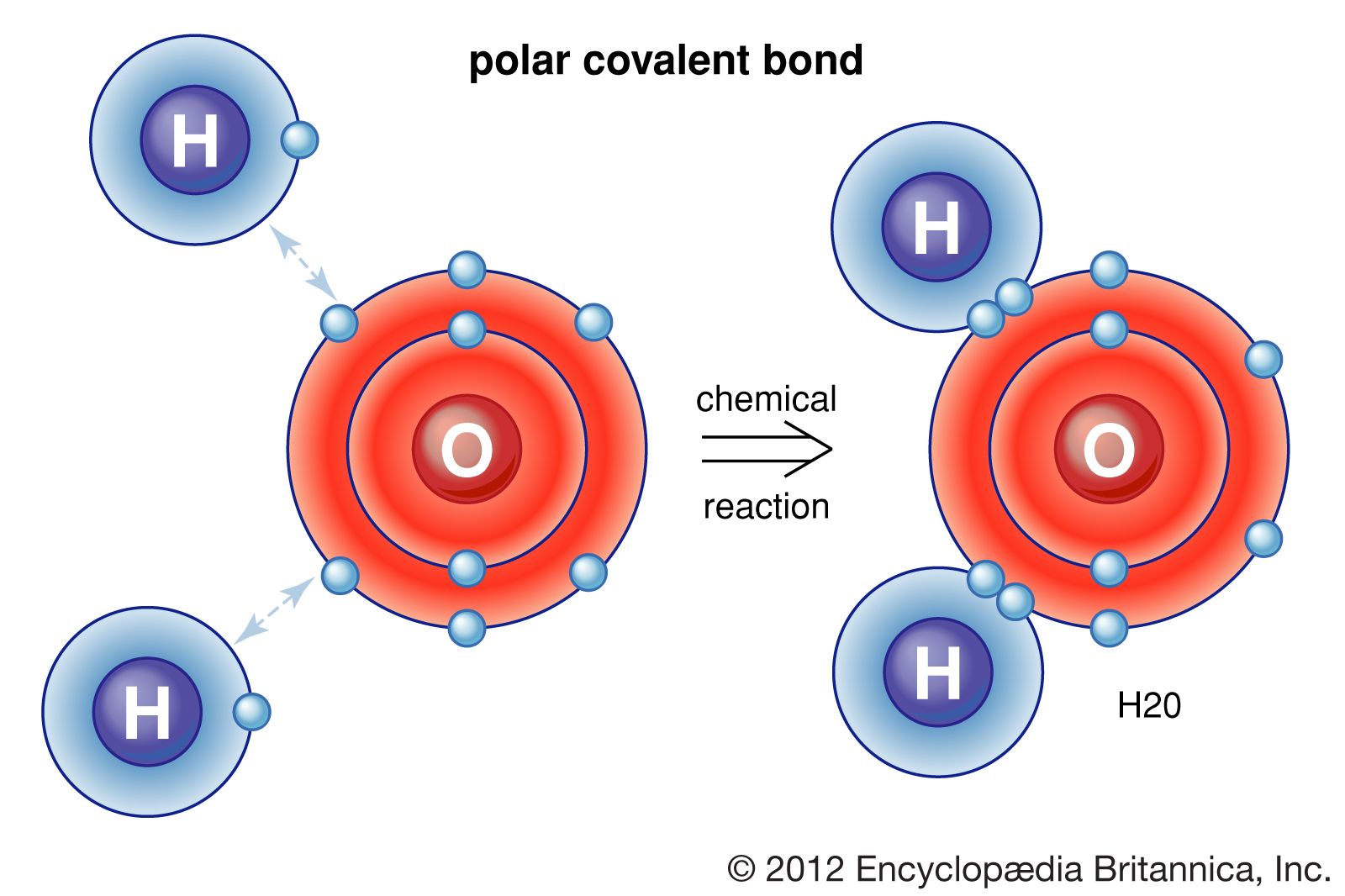
Covalent bonds, on the other hand, are formed when two or more atoms share one or more pairs of electrons to achieve a stable electronic configuration. Covalent bonds can be polar or nonpolar, depending on the difference in electronegativity between the atoms involved. Covalent bonds are typically found in molecules that consist of nonmetals.
5 Ways to Master Ionic and Covalent Bonds Coloring
Mastering ionic and covalent bonds coloring requires a combination of theoretical knowledge and practical application. Here are five ways to help you achieve mastery:
1. Understand the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. Understanding the octet rule is essential for predicting the type of bond that will form between atoms. By applying the octet rule, you can determine whether an atom will form an ionic or covalent bond.
2. Learn to Identify Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. By learning to identify electronegativity values, you can predict the type of bond that will form between atoms. In general, if the difference in electronegativity between two atoms is greater than 1.7, an ionic bond will form. If the difference is less than 1.7, a covalent bond will form.
3. Practice Drawing Lewis Structures
Lewis structures are a graphical representation of the electrons in a molecule. By practicing drawing Lewis structures, you can visualize the bonding between atoms and predict the type of bond that will form. Lewis structures can also help you identify the polarity of covalent bonds.
4. Use Color-Coding to Identify Bonds
Color-coding is a useful technique for identifying ionic and covalent bonds. By using different colors to represent ionic and covalent bonds, you can quickly identify the type of bond that is present in a molecule. For example, you can use red to represent ionic bonds and blue to represent covalent bonds.
| Bond Type | Color Code |
|---|---|
| Ionic Bond | Red |
| Covalent Bond | Blue |
5. Use Online Resources to Practice
There are many online resources available to help you practice identifying ionic and covalent bonds. Websites such as Khan Academy and ChemTube3D offer interactive tutorials and practice exercises to help you master ionic and covalent bonds coloring.
🔥 Note: Practice is key to mastering ionic and covalent bonds coloring. Make sure to practice regularly and use a variety of online resources to reinforce your learning.
As you can see, mastering ionic and covalent bonds coloring requires a combination of theoretical knowledge and practical application. By following these five tips, you can improve your understanding of chemical bonding and become proficient in identifying ionic and covalent bonds.
In conclusion, ionic and covalent bonds are the building blocks of molecules, and understanding their coloring is essential for any chemistry student. By mastering ionic and covalent bonds coloring, you can unlock the secrets of chemical bonding and gain a deeper understanding of the molecular world.
What is the main difference between ionic and covalent bonds?
+The main difference between ionic and covalent bonds is the way in which electrons are shared or transferred between atoms. Ionic bonds involve the transfer of electrons, resulting in the formation of ions with opposite charges, while covalent bonds involve the sharing of electrons between atoms.
How can I practice identifying ionic and covalent bonds?
+There are many online resources available to help you practice identifying ionic and covalent bonds, including Khan Academy and ChemTube3D. You can also practice drawing Lewis structures and using color-coding to identify bonds.
What is electronegativity, and how does it affect bonding?
+Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. The difference in electronegativity between two atoms can predict the type of bond that will form. If the difference is greater than 1.7, an ionic bond will form. If the difference is less than 1.7, a covalent bond will form.