5 Essential Steps to Master Chemical Reactions

Mastering Chemical Reactions: A Fundamental Guide
Chemical reactions are the building blocks of chemistry, and understanding them is crucial for any aspiring chemist or science enthusiast. From the simplest combustion reactions to complex organic synthesis, mastering chemical reactions requires a deep understanding of the underlying principles and mechanisms. In this article, we will explore the 5 essential steps to master chemical reactions and provide a comprehensive guide to help you navigate the world of chemistry.
Step 1: Understand the Basics of Chemical Reactions
Before diving into the intricacies of chemical reactions, it’s essential to grasp the fundamental concepts. A chemical reaction is a process in which one or more substances (reactants) are converted into new substances (products). Chemical reactions involve the breaking and forming of chemical bonds, which requires energy.
Types of Chemical Reactions:
- Synthesis reactions: Two or more reactants combine to form a new product.
- Decomposition reactions: A single reactant breaks down into two or more products.
- Replacement reactions: One reactant replaces another reactant in a compound.
- Combustion reactions: A reactant combines with oxygen to produce heat and light.
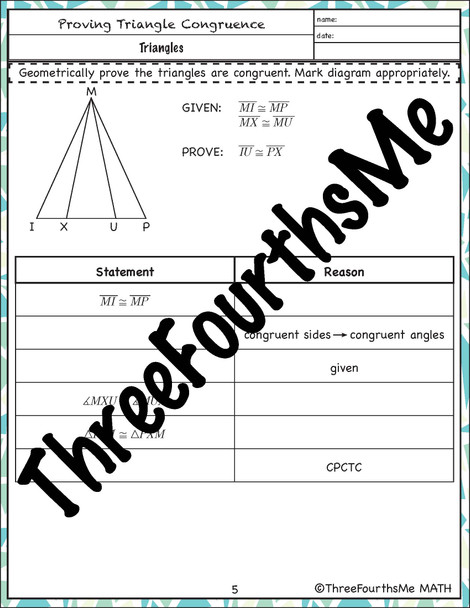
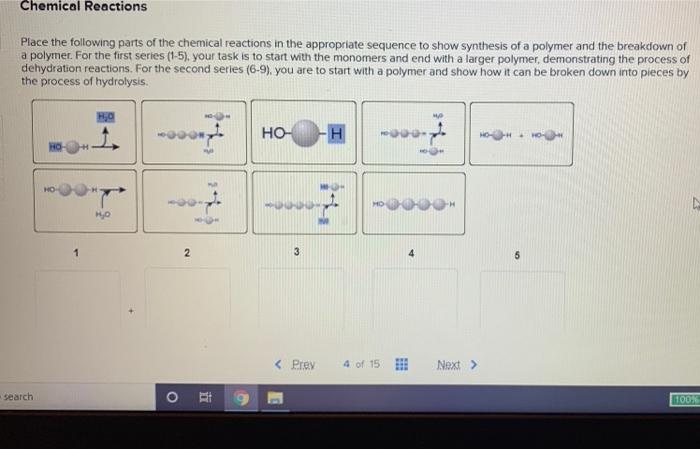
Step 2: Learn to Write and Balance Chemical Equations
Writing and balancing chemical equations is a critical skill in mastering chemical reactions. A chemical equation represents the reactants, products, and direction of the reaction. To write a balanced equation, ensure that the number of atoms of each element is the same on both the reactant and product sides.
Tips for Writing and Balancing Chemical Equations:
- Write the reactants on the left side of the equation and the products on the right side.
- Use arrows to indicate the direction of the reaction.
- Balance the equation by adding coefficients (numbers in front of the formulas of reactants or products).
- Check the equation to ensure that the number of atoms of each element is the same on both sides.
| Reactants | Products |
|---|---|
| 2H2 + O2 | 2H2O |
Step 3: Understand the Principles of Stoichiometry
Stoichiometry is the quantitative relationship between the reactants and products in a chemical reaction. It’s essential to understand the principles of stoichiometry to predict the amount of products formed or the amount of reactants required.
Key Concepts in Stoichiometry:
- Mole ratio: The ratio of moles of one substance to moles of another substance.
- Limiting reagent: The reactant that is consumed first in a reaction.
- Percent yield: The percentage of the theoretical yield that is actually obtained.
Step 4: Explore Reaction Mechanisms and Kinetics
Reaction mechanisms and kinetics are crucial in understanding the rate and efficiency of chemical reactions. Reaction mechanisms describe the step-by-step process of a reaction, while kinetics studies the rate of reaction.
Key Concepts in Reaction Mechanisms and Kinetics:
- Reaction rate: The rate at which reactants are converted into products.
- Rate-determining step: The slowest step in a reaction mechanism.
- Activation energy: The energy required for a reaction to occur.
Step 5: Practice and Apply Your Knowledge
The final step in mastering chemical reactions is to practice and apply your knowledge. Practice problems, simulations, and laboratory experiments can help reinforce your understanding of chemical reactions.
Tips for Practicing and Applying Your Knowledge:
- Practice writing and balancing chemical equations.
- Solve stoichiometry problems to predict the amount of products formed.
- Research and explore different reaction mechanisms and kinetics.
- Participate in laboratory experiments to apply your knowledge in real-world scenarios.
💡 Note: Mastering chemical reactions requires patience, persistence, and practice. Don't be discouraged if you struggle at first – with time and effort, you'll become proficient in writing and balancing chemical equations, understanding stoichiometry, and exploring reaction mechanisms and kinetics.
In conclusion, mastering chemical reactions requires a deep understanding of the underlying principles and mechanisms. By following these 5 essential steps, you’ll be well on your way to becoming proficient in chemistry and unlocking the secrets of chemical reactions.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction involves the combination of two or more reactants to form a new product, while a decomposition reaction involves the breakdown of a single reactant into two or more products.
What is the purpose of balancing chemical equations?
+Balancing chemical equations ensures that the number of atoms of each element is the same on both the reactant and product sides, which is a fundamental principle of chemistry.
What is the difference between reaction mechanisms and kinetics?
+Reaction mechanisms describe the step-by-step process of a reaction, while kinetics studies the rate of reaction and the factors that affect it.