Identifying Chemical Reactions Worksheet Answers
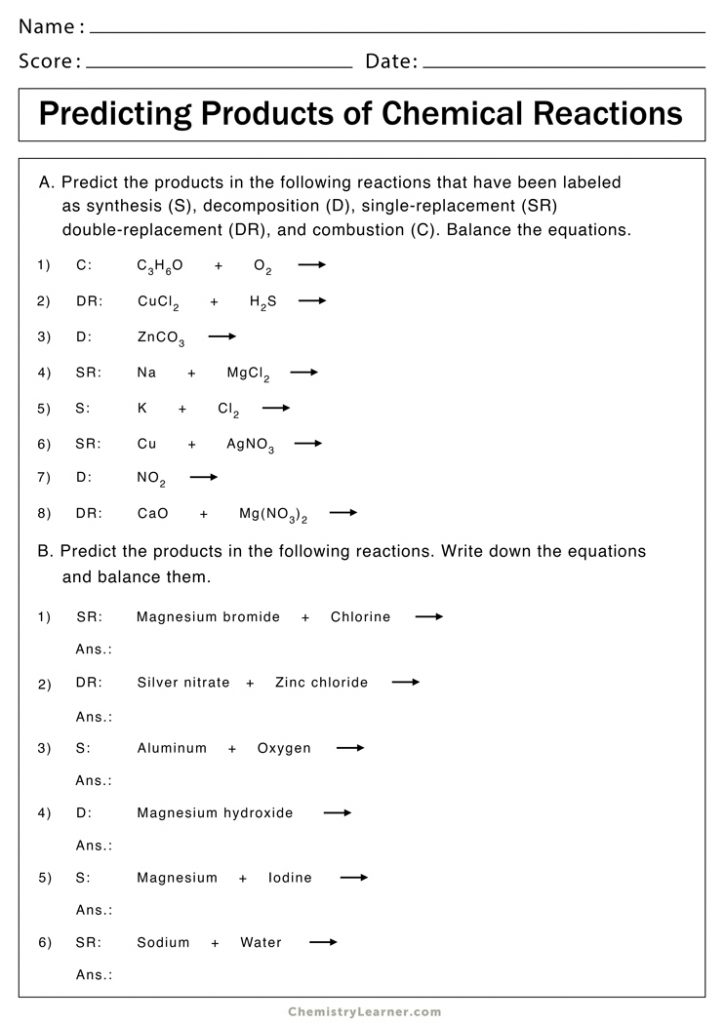
Introduction to Chemical Reactions
Chemical reactions are processes in which one or more substances are converted into new substances. These reactions involve the breaking and forming of chemical bonds between atoms, resulting in changes to the chemical composition of the substances involved. Understanding chemical reactions is crucial in various fields, including chemistry, biology, and environmental science.
Types of Chemical Reactions
There are several types of chemical reactions, including:
- Synthesis reactions: Two or more substances combine to form a new compound.
- Decomposition reactions: A single compound breaks down into two or more simpler substances.
- Replacement reactions: One element takes the place of another element in a compound.
- Combustion reactions: A substance reacts with oxygen to produce heat and light.
Identifying Chemical Reactions
To identify a chemical reaction, look for the following characteristics:
- Change in color: A change in color can indicate a chemical reaction.
- Release of gas: The release of a gas can indicate a chemical reaction.
- Formation of a precipitate: The formation of a solid from a solution can indicate a chemical reaction.
- Change in temperature: A change in temperature can indicate a chemical reaction.
Worksheet Answers
Here are the answers to a sample worksheet on identifying chemical reactions:
1. What type of reaction is represented by the equation:
2Na (s) + Cl2 (g) → 2NaCl (s)
- Answer: Synthesis reaction
2. Which of the following is an example of a decomposition reaction?
- Answer: 2H2O (l) → 2H2 (g) + O2 (g)
3. What type of reaction is represented by the equation:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
- Answer: Replacement reaction
4. Which of the following is an example of a combustion reaction?
- Answer: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
5. What is the type of reaction that occurs when a solid forms from a solution?
- Answer: Precipitation reaction
👍 Note: A precipitation reaction is a type of chemical reaction that occurs when a solid forms from a solution.
Conclusion
In conclusion, identifying chemical reactions is an essential skill in chemistry and other sciences. By recognizing the characteristics of different types of reactions, such as synthesis, decomposition, replacement, and combustion reactions, you can better understand the chemical processes that occur around us.
What is a chemical reaction?
+A chemical reaction is a process in which one or more substances are converted into new substances.
What are the different types of chemical reactions?
+There are several types of chemical reactions, including synthesis, decomposition, replacement, and combustion reactions.
How can you identify a chemical reaction?
+Look for changes in color, release of gas, formation of a precipitate, or change in temperature.
Related Terms:
- Types of chemical reactions Notes