Master 5 Steps to Naming Binary Ionic Compounds

Understanding Binary Ionic Compounds
Binary ionic compounds are composed of two elements: a metal and a nonmetal. These compounds are formed when one or more electrons are transferred from the metal atom to the nonmetal atom, resulting in the formation of ions with opposite charges. The electrostatic attraction between these oppositely charged ions holds the compound together.
Step 1: Identify the Cation and Anion
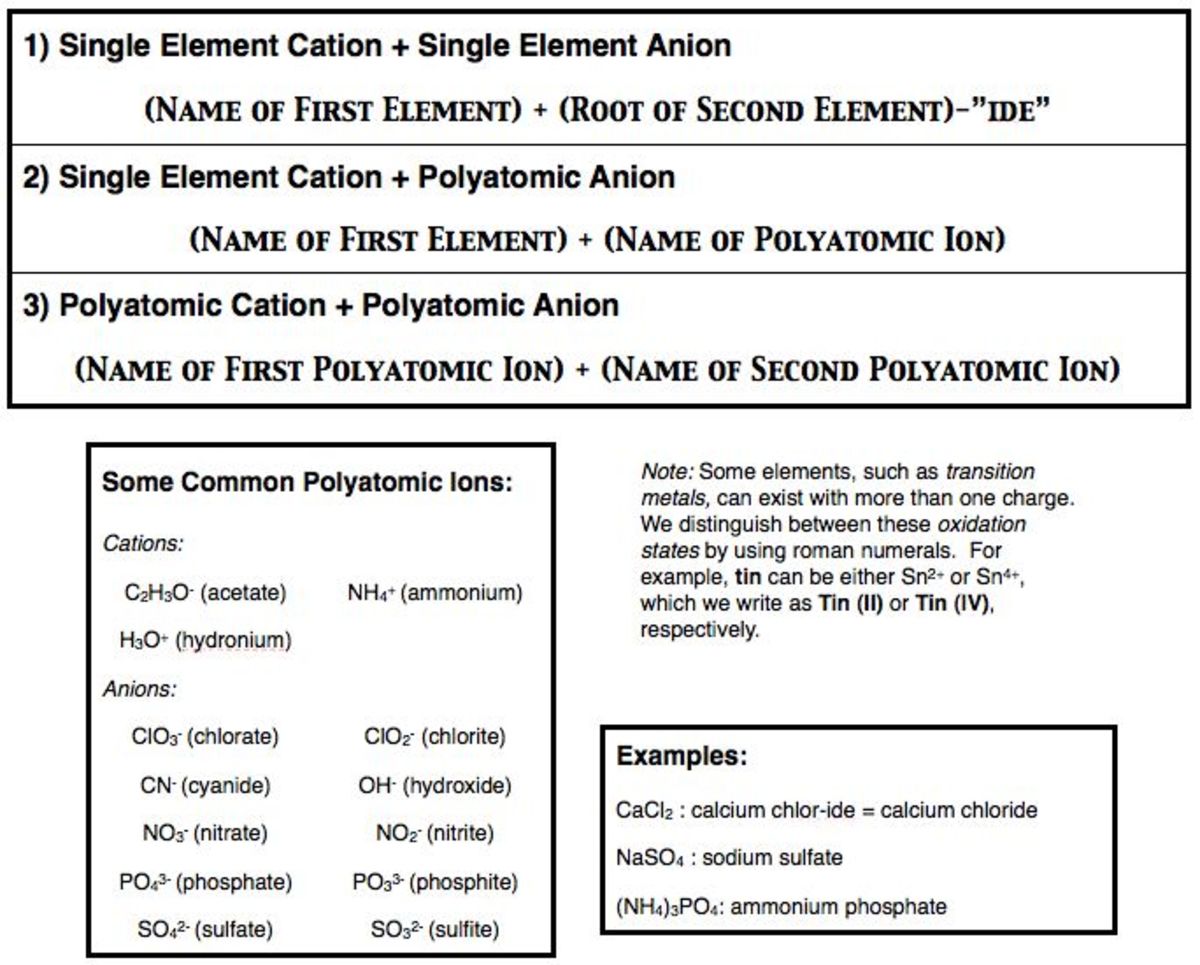
To name a binary ionic compound, you first need to identify the cation (positive ion) and the anion (negative ion). The cation is usually the metal, and the anion is the nonmetal. For example, in the compound NaCl, Na+ is the cation, and Cl- is the anion.
🔥 Note: Metals are typically found on the left side of the periodic table, while nonmetals are on the right side.
Step 2: Determine the Charge of the Cation and Anion
The charge of the cation and anion can be determined by looking at the periodic table. Metals tend to lose electrons to form positive ions, while nonmetals tend to gain electrons to form negative ions. The charge of the cation is typically equal to the number of electrons lost, while the charge of the anion is equal to the number of electrons gained.
For example, in the compound NaCl, the sodium atom (Na) loses one electron to form a +1 ion, while the chlorine atom (Cl) gains one electron to form a -1 ion.
Step 3: Write the Name of the Cation
The name of the cation is the same as the name of the metal. For example, in the compound NaCl, the cation is sodium.
If the metal can form ions with different charges, the charge of the cation is indicated in parentheses. For example, iron can form +2 and +3 ions, so the compound FeCl2 would be named iron(II) chloride.
Step 4: Write the Name of the Anion
The name of the anion is the root of the nonmetal’s name, followed by the suffix “-ide”. For example, in the compound NaCl, the anion is chloride.
If the nonmetal can form ions with different charges, the charge of the anion is indicated by changing the suffix. For example, oxygen can form -1 and -2 ions, so the compound Na2O would be named sodium oxide.
Step 5: Combine the Names of the Cation and Anion
The final step is to combine the names of the cation and anion to form the name of the binary ionic compound. The cation is named first, followed by the anion.
For example, the compound NaCl would be named sodium chloride.
📝 Note: When writing the name of a binary ionic compound, make sure to use the correct suffix for the anion.
| Compound | Name of Cation | Name of Anion | Name of Compound |
|---|---|---|---|
| NaCl | Sodium | Chloride | Sodium chloride |
| CaO | Calcium | Oxide | Calcium oxide |
| FeCl2 | Iron(II) | Chloride | Iron(II) chloride |
By following these five steps, you can easily name binary ionic compounds.
In summary, naming binary ionic compounds involves identifying the cation and anion, determining their charges, writing the name of the cation and anion, and combining them to form the name of the compound. With practice, you’ll become proficient in naming these compounds.
What is the difference between a cation and an anion?
+A cation is a positively charged ion, typically formed when a metal atom loses one or more electrons. An anion is a negatively charged ion, typically formed when a nonmetal atom gains one or more electrons.
How do I determine the charge of a cation or anion?
+The charge of a cation or anion can be determined by looking at the periodic table. Metals tend to lose electrons to form positive ions, while nonmetals tend to gain electrons to form negative ions.
What is the correct suffix for an anion?
+The correct suffix for an anion is “-ide”. For example, the anion formed by chlorine is called chloride.