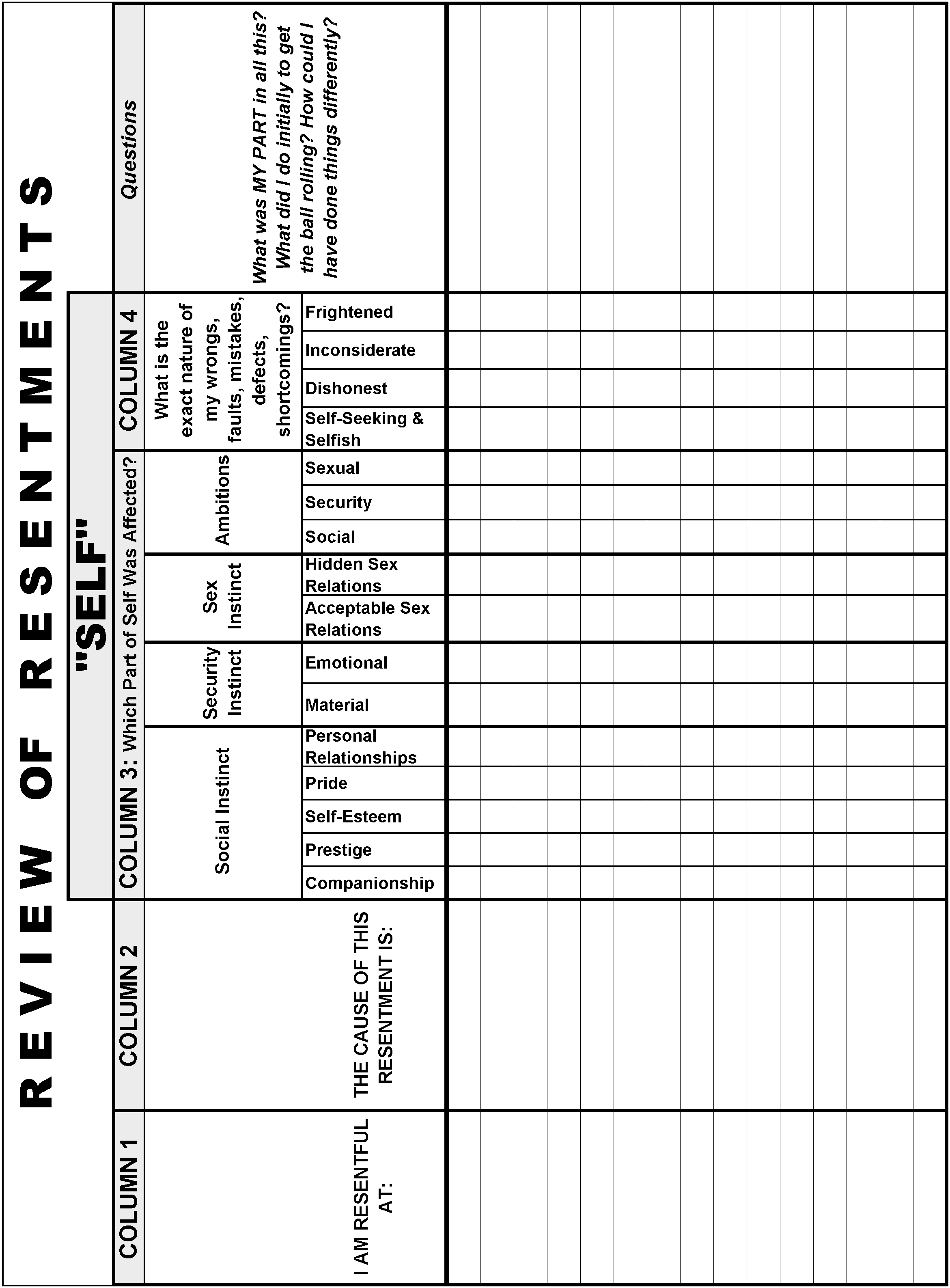
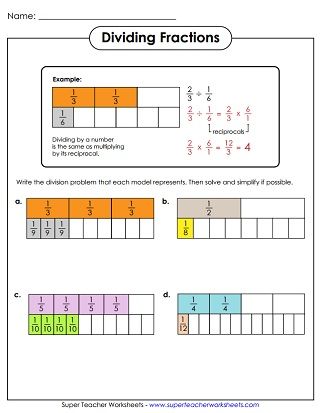
5 Ways to Master Empirical Formula Worksheet Answers
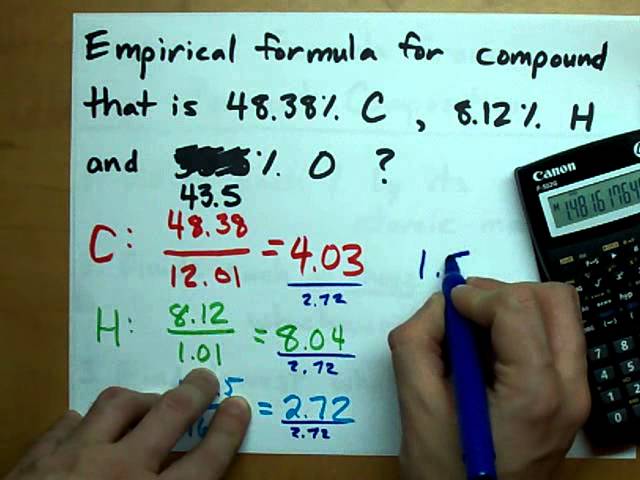
Mastering Empirical Formula Worksheet Answers: A Comprehensive Guide
Empirical formulas are a fundamental concept in chemistry, representing the simplest whole-number ratio of atoms of each element present in a compound. Mastering empirical formulas is crucial for chemistry students, and worksheet answers can be an excellent resource to help them achieve this goal. In this article, we will explore five ways to master empirical formula worksheet answers, including understanding the concept, breaking down problems, using online resources, practicing with sample problems, and seeking help when needed.
Understanding Empirical Formulas
Before diving into worksheet answers, it’s essential to understand the concept of empirical formulas. An empirical formula represents the simplest whole-number ratio of atoms of each element present in a compound. It’s calculated by dividing the number of moles of each element by the greatest common divisor (GCD) of the number of moles.
For example, if a compound contains 3 moles of carbon, 4 moles of hydrogen, and 1 mole of oxygen, the empirical formula would be CH4O.
📝 Note: Empirical formulas do not provide information about the actual number of atoms of each element in a molecule, but rather the simplest ratio.
Breaking Down Problems
To master empirical formula worksheet answers, it’s crucial to break down problems into manageable steps. Here’s a step-by-step approach:
- Step 1: Read the problem carefully and identify the given information, such as the number of moles of each element.
- Step 2: Calculate the greatest common divisor (GCD) of the number of moles.
- Step 3: Divide the number of moles of each element by the GCD.
- Step 4: Write the empirical formula using the resulting ratios.
By following these steps, you’ll be able to tackle even the most challenging empirical formula problems.
Using Online Resources
There are numerous online resources available to help you master empirical formula worksheet answers. Some popular options include:
- Khan Academy: Khan Academy offers a comprehensive tutorial on empirical formulas, including video lessons and practice exercises.
- ChemGuide: ChemGuide provides a detailed explanation of empirical formulas, including examples and practice problems.
- Empirical Formula Calculator: This online calculator allows you to input the number of moles of each element and calculates the empirical formula.
By utilizing these resources, you’ll be able to supplement your learning and gain a deeper understanding of empirical formulas.
Practicing with Sample Problems
Practice is key to mastering empirical formula worksheet answers. Here are a few sample problems to get you started:
- Problem 1: A compound contains 2 moles of carbon, 3 moles of hydrogen, and 1 mole of oxygen. What is the empirical formula?
- Problem 2: A compound contains 4 moles of nitrogen, 2 moles of hydrogen, and 1 mole of oxygen. What is the empirical formula?
By practicing with sample problems, you’ll become more comfortable with the process of calculating empirical formulas.
Seeking Help When Needed
Finally, don’t be afraid to seek help when needed. If you’re struggling with empirical formula worksheet answers, consider:
- Asking your teacher: Your teacher can provide additional guidance and support to help you understand the concept.
- Joining a study group: Joining a study group can provide an opportunity to collaborate with classmates and learn from one another.
- Seeking online tutoring: Online tutoring services can provide one-on-one support and guidance.
By seeking help when needed, you’ll be able to overcome any challenges and achieve mastery of empirical formula worksheet answers.
In conclusion, mastering empirical formula worksheet answers requires a combination of understanding the concept, breaking down problems, using online resources, practicing with sample problems, and seeking help when needed. By following these steps, you’ll be well on your way to achieving success in chemistry.
What is the difference between a molecular formula and an empirical formula?
+A molecular formula represents the actual number of atoms of each element in a molecule, while an empirical formula represents the simplest whole-number ratio of atoms of each element.
How do I calculate the greatest common divisor (GCD) of the number of moles?
+To calculate the GCD, list the factors of each number and identify the greatest common factor.
What online resources are available to help me master empirical formula worksheet answers?
+Some popular online resources include Khan Academy, ChemGuide, and Empirical Formula Calculator.
Related Terms:
- Empirical/molecular formula Practice Worksheet answers
- Empirical and molecular formula Worksheet