Empirical and Molecular Formulas Made Easy

Understanding Empirical and Molecular Formulas
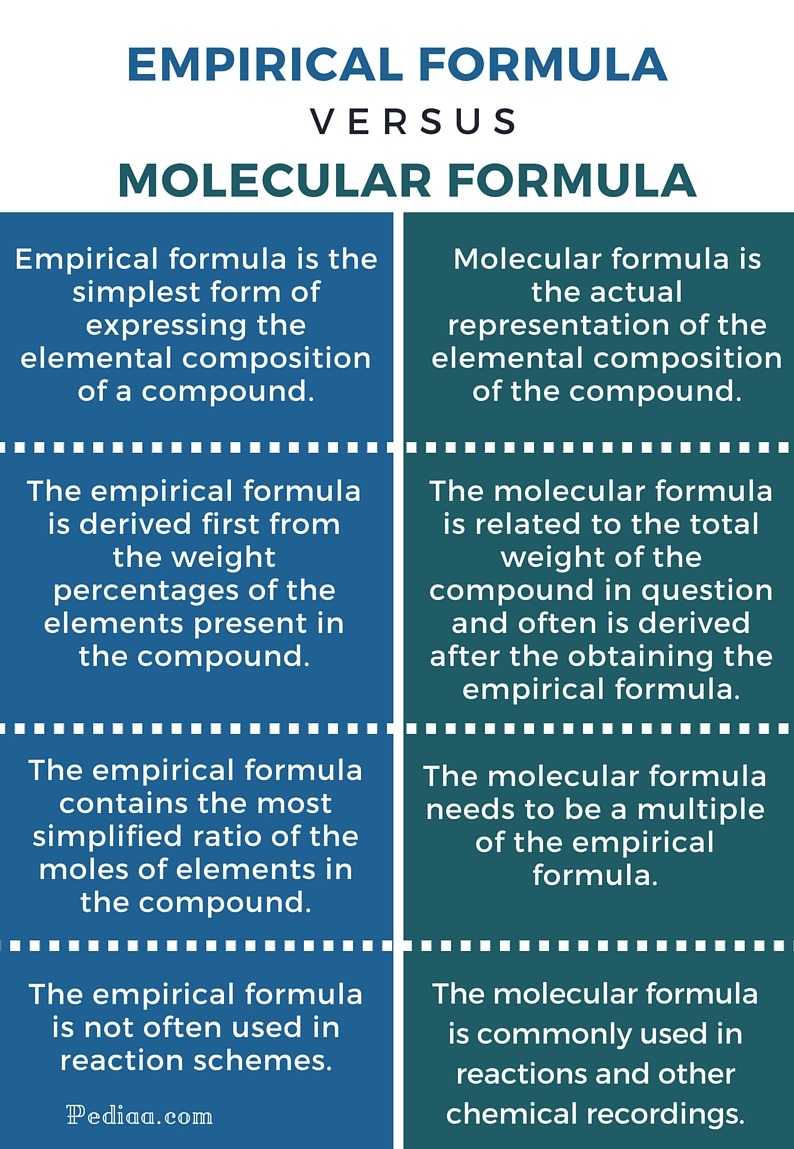
In chemistry, the empirical and molecular formulas are two types of formulas that provide crucial information about the composition of a molecule. While they may seem complex, understanding the differences between them can be made easy with the right approach. In this article, we will delve into the world of empirical and molecular formulas, exploring their definitions, differences, and how to calculate them.
What is an Empirical Formula?
An empirical formula is the simplest whole-number ratio of atoms of each element present in a compound. It is a concise way of expressing the composition of a molecule without providing information about the actual number of atoms or the arrangement of atoms. Empirical formulas are calculated based on the mass percent composition of a compound, which is the percentage of each element by mass.
For example, the empirical formula for glucose (C6H12O6) is CH2O. This formula indicates that glucose is composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio, but it does not provide information about the actual number of atoms or the arrangement of atoms.
What is a Molecular Formula?
A molecular formula, on the other hand, is a formula that represents the actual number of atoms of each element present in a molecule. It is a more detailed representation of a molecule’s composition compared to an empirical formula. Molecular formulas are calculated based on the molecular weight of a compound, which is the sum of the atomic weights of all the atoms in the molecule.
Using the same example as before, the molecular formula for glucose is C6H12O6. This formula indicates that a molecule of glucose is composed of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Key Differences Between Empirical and Molecular Formulas
So, what are the key differences between empirical and molecular formulas? Here are some key points to note:
- Simplicity: Empirical formulas are simpler and more concise, representing the simplest whole-number ratio of atoms. Molecular formulas, on the other hand, represent the actual number of atoms.
- Information: Empirical formulas provide information about the composition of a molecule, but not the actual number of atoms. Molecular formulas provide information about the actual number of atoms and the arrangement of atoms.
- Calculation: Empirical formulas are calculated based on the mass percent composition of a compound, while molecular formulas are calculated based on the molecular weight of a compound.
🔍 Note: Empirical formulas are often used as a starting point for calculating molecular formulas, as they provide a simplified representation of a molecule's composition.
Calculating Empirical Formulas
Calculating empirical formulas involves determining the mass percent composition of a compound and then converting it to a whole-number ratio of atoms. Here’s a step-by-step guide to calculating empirical formulas:
- Determine the mass percent composition: Calculate the mass percent composition of a compound by dividing the mass of each element by the total mass of the compound and multiplying by 100.
- Convert to a whole-number ratio: Convert the mass percent composition to a whole-number ratio of atoms by dividing each percentage by the atomic weight of the element.
- Simplify the ratio: Simplify the ratio to the simplest whole-number ratio of atoms.
For example, let’s calculate the empirical formula for a compound composed of 40% carbon, 30% hydrogen, and 30% oxygen by mass.
| Element | Mass Percent | Atomic Weight | Whole-Number Ratio |
|---|---|---|---|
| Carbon | 40% | 12 | 3.33 |
| Hydrogen | 30% | 1 | 30 |
| Oxygen | 30% | 16 | 1.88 |
To simplify the ratio, divide each number by the smallest number:
| Element | Simplified Ratio |
|---|---|
| Carbon | 1.67 |
| Hydrogen | 15 |
| Oxygen | 0.94 |
Rounding the numbers to the nearest whole number, we get:
| Element | Empirical Formula |
|---|---|
| Carbon | 1 |
| Hydrogen | 8 |
| Oxygen | 1 |
The empirical formula for the compound is CH8O.
Calculating Molecular Formulas
Calculating molecular formulas involves determining the molecular weight of a compound and then converting it to a formula that represents the actual number of atoms. Here’s a step-by-step guide to calculating molecular formulas:
- Determine the molecular weight: Calculate the molecular weight of a compound by summing the atomic weights of all the atoms in the molecule.
- Determine the empirical formula: Calculate the empirical formula of the compound using the method described earlier.
- Determine the multiplier: Calculate the multiplier by dividing the molecular weight by the empirical formula weight.
- Calculate the molecular formula: Multiply the empirical formula by the multiplier to get the molecular formula.
For example, let’s calculate the molecular formula for glucose (C6H12O6) using the empirical formula CH2O.
- Determine the molecular weight: The molecular weight of glucose is 180 g/mol.
- Determine the empirical formula: The empirical formula for glucose is CH2O.
- Determine the multiplier: Divide the molecular weight by the empirical formula weight:
Multiplier = Molecular Weight / Empirical Formula Weight = 180 g/mol / 30 g/mol = 6
- Calculate the molecular formula: Multiply the empirical formula by the multiplier:
Molecular Formula = Empirical Formula x Multiplier = CH2O x 6 = C6H12O6
The molecular formula for glucose is C6H12O6.
🔍 Note: Molecular formulas can be used to determine the actual number of atoms in a molecule, which is useful in calculating the number of moles of a substance.
What is the difference between an empirical formula and a molecular formula?
+An empirical formula is the simplest whole-number ratio of atoms of each element present in a compound, while a molecular formula represents the actual number of atoms of each element present in a molecule.
How do I calculate the empirical formula of a compound?
+To calculate the empirical formula, determine the mass percent composition of a compound, convert it to a whole-number ratio of atoms, and simplify the ratio to the simplest whole-number ratio of atoms.
What is the purpose of calculating molecular formulas?
+Molecular formulas are used to determine the actual number of atoms in a molecule, which is useful in calculating the number of moles of a substance.
In conclusion, understanding the differences between empirical and molecular formulas is crucial in chemistry. By mastering the calculations and concepts, you can easily determine the composition of a molecule and calculate the number of moles of a substance.