5 Essential Concepts: Elements, Compounds, and Mixtures
Understanding the Basics of Chemistry: Elements, Compounds, and Mixtures
Chemistry is a vast and fascinating field that plays a vital role in our daily lives. From the food we eat to the air we breathe, chemistry is involved in every aspect of our lives. However, to appreciate the complexity and beauty of chemistry, it is essential to understand the fundamental concepts that form the basis of this field. In this article, we will explore three critical concepts: elements, compounds, and mixtures.
Elements: The Building Blocks of Chemistry
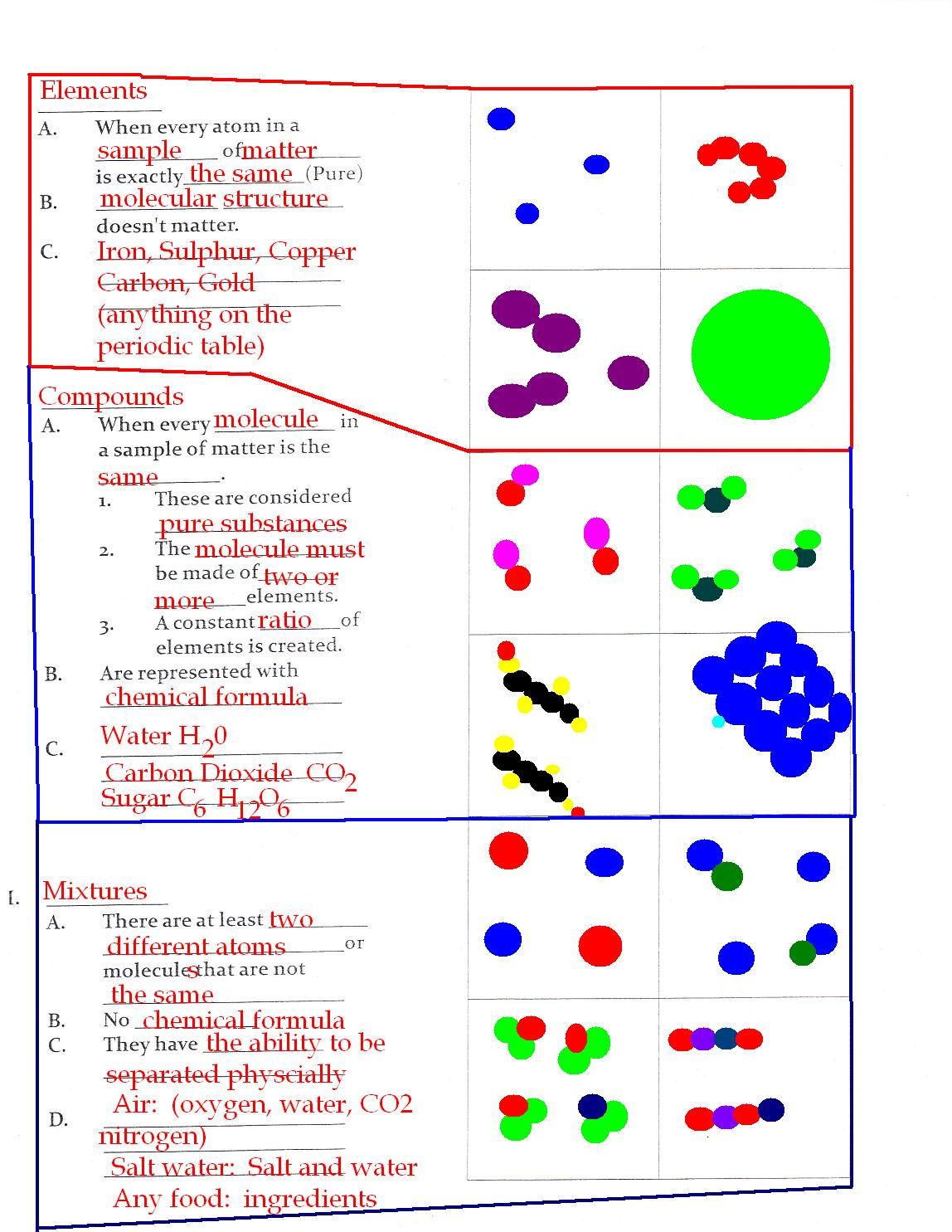
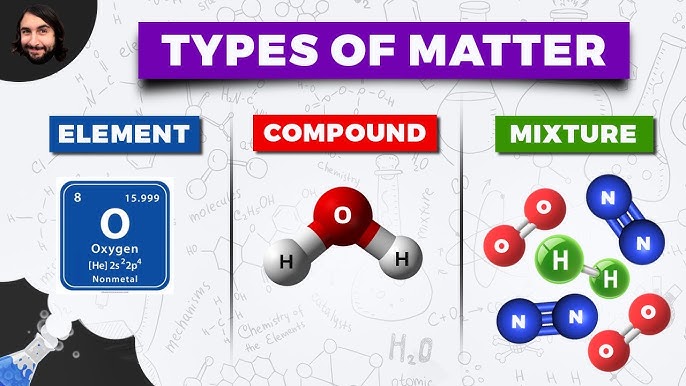
Elements are the simplest substances in chemistry. They are substances that consist of only one type of atom and cannot be broken down into simpler substances by chemical means. In other words, elements are the basic building blocks of chemistry. There are 118 known elements, each with its unique properties and characteristics.
Elements are represented by symbols, which are usually one or two-letter abbreviations of the element’s name. For example, the symbol for hydrogen is H, while the symbol for oxygen is O. Elements can be further divided into two categories: metals and non-metals. Metals are typically shiny, malleable, and good conductors of electricity, while non-metals are dull, brittle, and poor conductors of electricity.
🔬 Note: Elements can be found naturally or synthesized in a laboratory. However, synthesized elements are often unstable and decay quickly into more stable elements.
Compounds: The Combination of Elements
Compounds are substances formed by the chemical combination of two or more different elements. In other words, compounds are made up of atoms of different elements that are chemically bonded together. Compounds have properties that are different from those of their individual elements.
For example, water (H2O) is a compound made up of two hydrogen atoms and one oxygen atom. The properties of water are different from those of hydrogen and oxygen. While hydrogen is a flammable gas, and oxygen is a gas that supports combustion, water is a liquid that extinguishes fires.
Compounds can be further divided into two categories: molecular compounds and ionic compounds. Molecular compounds are formed by the sharing of electrons between atoms, while ionic compounds are formed by the transfer of electrons between atoms.
Mixtures: The Combination of Substances
Mixtures are physical combinations of two or more substances that are not chemically bonded together. In other words, mixtures are made up of substances that are not chemically reacted with each other. Mixtures have properties that are a combination of the properties of their individual components.
For example, air is a mixture of gases, including nitrogen, oxygen, and carbon dioxide. Each gas in the mixture retains its individual properties, and the mixture as a whole has properties that are a combination of the properties of its individual components.
Mixtures can be further divided into two categories: homogeneous mixtures and heterogeneous mixtures. Homogeneous mixtures are mixtures in which the components are evenly distributed, while heterogeneous mixtures are mixtures in which the components are not evenly distributed.
| Element | Compound | Mixture |
|---|---|---|
| Single type of atom | Chemical combination of elements | Physical combination of substances |
| Cannot be broken down into simpler substances | Has properties different from individual elements | Has properties that are a combination of individual components |
Conclusion
In conclusion, elements, compounds, and mixtures are three essential concepts in chemistry. Understanding these concepts is crucial for appreciating the complexity and beauty of chemistry. By recognizing the differences between elements, compounds, and mixtures, we can better understand the world around us and the chemical reactions that occur in our daily lives.
Recap
- Elements are the simplest substances in chemistry, consisting of only one type of atom.
- Compounds are substances formed by the chemical combination of two or more different elements.
- Mixtures are physical combinations of two or more substances that are not chemically bonded together.
What is the difference between an element and a compound?
+An element is a substance that consists of only one type of atom, while a compound is a substance formed by the chemical combination of two or more different elements.
What is a mixture?
+A mixture is a physical combination of two or more substances that are not chemically bonded together.
What are the two categories of compounds?
+Compounds can be divided into two categories: molecular compounds and ionic compounds.
Related Terms:
- Elements and compounds Worksheet PDF