Electronegativity Worksheet Answers: Mastering Chemistry's Fundamental Concept
Understanding Electronegativity: A Fundamental Concept in Chemistry
Electronegativity is a crucial concept in chemistry that helps us understand the behavior of atoms in a molecule. It is a measure of an atom’s ability to attract electrons in a covalent bond. In this article, we will explore the concept of electronegativity, its importance, and provide a comprehensive electronegativity worksheet with answers.
What is Electronegativity?
Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. It is a scale that ranges from 0 to 4.0, with higher values indicating a greater ability to attract electrons. The concept of electronegativity was first introduced by Linus Pauling in the 1930s and has since become a fundamental concept in chemistry.
Why is Electronegativity Important?
Electronegativity is important because it helps us understand the behavior of atoms in a molecule. It helps us predict the polarity of a bond, the shape of a molecule, and the reactivity of a molecule. Electronegativity is also used to predict the acid-base properties of a molecule.
How is Electronegativity Measured?
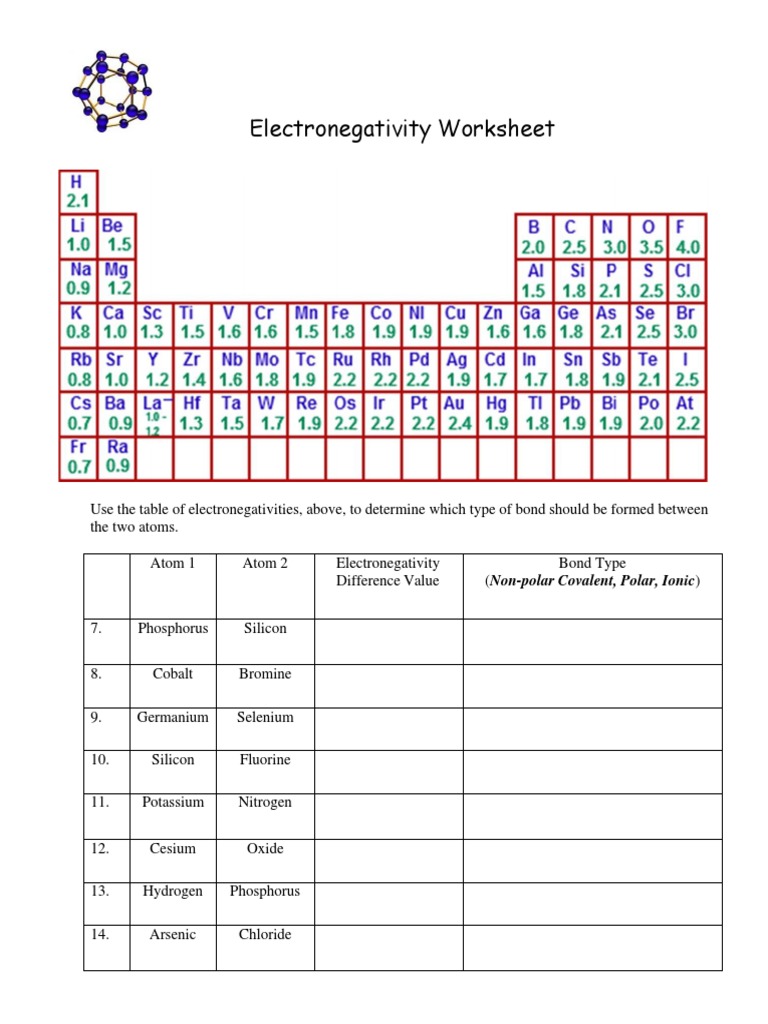
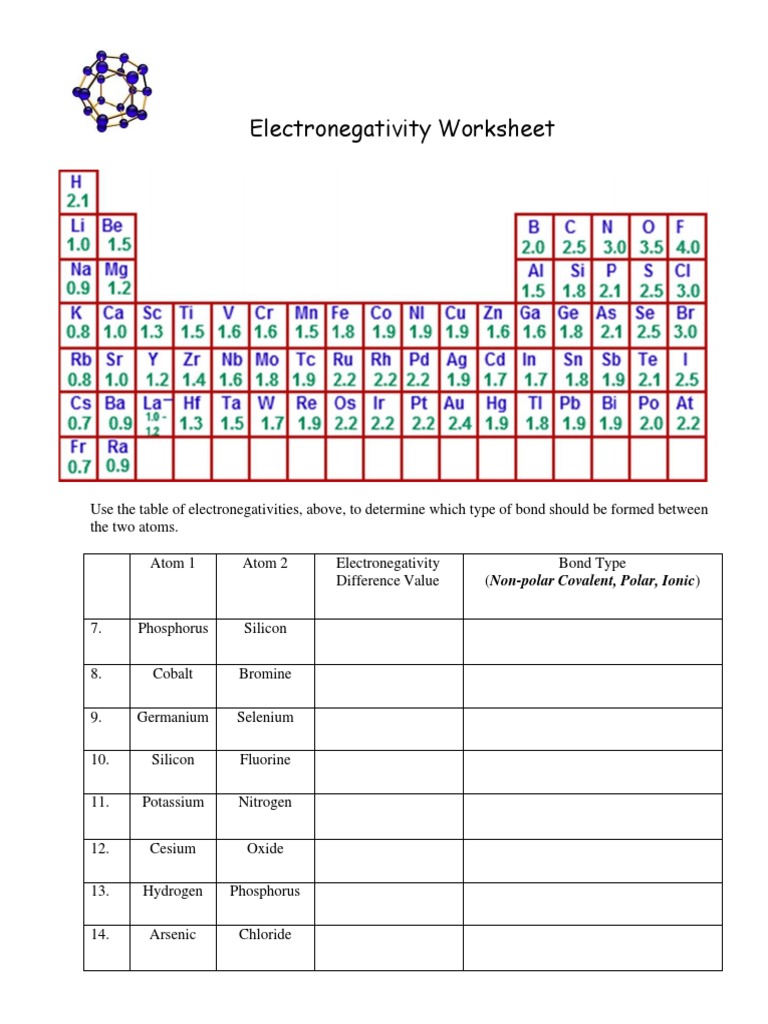
Electronegativity is measured using the Pauling scale, which ranges from 0 to 4.0. The Pauling scale is based on the electronegativity of hydrogen, which is set at 2.1. The electronegativity of other elements is then measured relative to hydrogen.
Electronegativity Values of Common Elements
Here are the electronegativity values of some common elements:
| Element | Electronegativity Value |
|---|---|
| Hydrogen | 2.1 |
| Oxygen | 3.4 |
| Nitrogen | 3.0 |
| Fluorine | 3.9 |
| Carbon | 2.5 |
| Sulfur | 2.5 |
| Phosphorus | 2.1 |
Electronegativity Worksheet Answers
Here are some examples of electronegativity problems, along with their answers:
Problem 1: What is the electronegativity value of chlorine?
Answer: 3.0
Problem 2: Which element has a higher electronegativity value, oxygen or nitrogen?
Answer: Oxygen (3.4) has a higher electronegativity value than nitrogen (3.0).
Problem 3: What is the electronegativity value of carbon?
Answer: 2.5
Problem 4: Which bond is more polar, a bond between oxygen and hydrogen or a bond between carbon and hydrogen?
Answer: A bond between oxygen and hydrogen is more polar because oxygen has a higher electronegativity value than carbon.
Electronegativity Trends
Electronegativity values follow certain trends:
- Electronegativity increases from left to right across a period: This is because the number of protons in the nucleus increases, which increases the attraction to electrons.
- Electronegativity decreases down a group: This is because the number of energy levels increases, which decreases the attraction to electrons.
Conclusion
Electronegativity is a fundamental concept in chemistry that helps us understand the behavior of atoms in a molecule. By understanding electronegativity, we can predict the polarity of a bond, the shape of a molecule, and the reactivity of a molecule. We hope this article and the electronegativity worksheet answers have helped you master this important concept.
What is the electronegativity value of fluorine?
+3.9
Which element has a higher electronegativity value, sulfur or phosphorus?
+Sulfur (2.5) and phosphorus (2.1) have similar electronegativity values, but sulfur has a slightly higher value.
What is the electronegativity trend across a period?
+Electronegativity increases from left to right across a period.
Related Terms:
- Electronegativity Worksheet answers PDF
- Electronegativity Worksheet pdf
- Difference in electronegativity Worksheet
- Predicting chemical bonds Worksheet answers