Master Electron Configuration in 5 Easy Steps
Unlocking the Secrets of Electron Configuration
Electron configuration is a fundamental concept in chemistry that can be intimidating at first, but with the right approach, it can be mastered in no time. In this article, we will break down the process of determining electron configuration into 5 easy steps. By the end of this tutorial, you will be able to confidently determine the electron configuration of any atom.
Step 1: Understand the Basics of Electron Configuration
Before we dive into the steps, it’s essential to understand what electron configuration is. Electron configuration is a way of describing the arrangement of electrons in an atom. It’s a shorthand notation that tells us which energy levels (or shells) the electrons occupy and how many electrons are in each energy level.
Key Concept: The Aufbau principle states that electrons occupy the lowest available energy levels.
Step 2: Determine the Atomic Number
The atomic number of an element is the number of protons in the nucleus of an atom. To determine the electron configuration, we need to know the atomic number of the element. This can be found on the periodic table.
Example: Let’s use the element Carbon © with an atomic number of 6.
Step 3: Fill in the Energy Levels
Using the Aufbau principle, we start filling in the energy levels with electrons. The energy levels are filled in the following order:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p,…
Example: For Carbon ©, we start filling in the energy levels:
1s², 2s², 2p²
👀 Note: The superscript number represents the number of electrons in each energy level.
Step 4: Use the Periodic Table to Determine the Electron Configuration
The periodic table is a powerful tool for determining electron configuration. By looking at the periodic table, we can determine which energy levels are filled and which are not.
Example: For Carbon ©, we look at the periodic table and see that it is in the second period (row) and group 14 (column). This tells us that the 2s and 2p energy levels are filled.
Electron Configuration: 1s², 2s², 2p²
Step 5: Check Your Work
To ensure that our electron configuration is correct, we need to check our work. We can do this by using the following steps:
- Count the number of electrons in the energy levels.
- Make sure that the number of electrons equals the atomic number.
- Check that the energy levels are filled in the correct order.
Example: For Carbon ©, we count the number of electrons:
1s² = 2 electrons 2s² = 2 electrons 2p² = 2 electrons
Total number of electrons: 2 + 2 + 2 = 6
This matches the atomic number of Carbon ©, which is 6.
By following these 5 easy steps, you can determine the electron configuration of any atom. Remember to always use the Aufbau principle and the periodic table to guide you.
Now, let’s summarize the key points:
- Electron configuration is a shorthand notation that describes the arrangement of electrons in an atom.
- The Aufbau principle states that electrons occupy the lowest available energy levels.
- The periodic table is a powerful tool for determining electron configuration.
- Always check your work by counting the number of electrons and making sure that the energy levels are filled in the correct order.
With practice, you will become proficient in determining electron configuration and will be able to tackle even the most challenging problems.
Here’s a table summarizing the electron configuration of the first 10 elements:
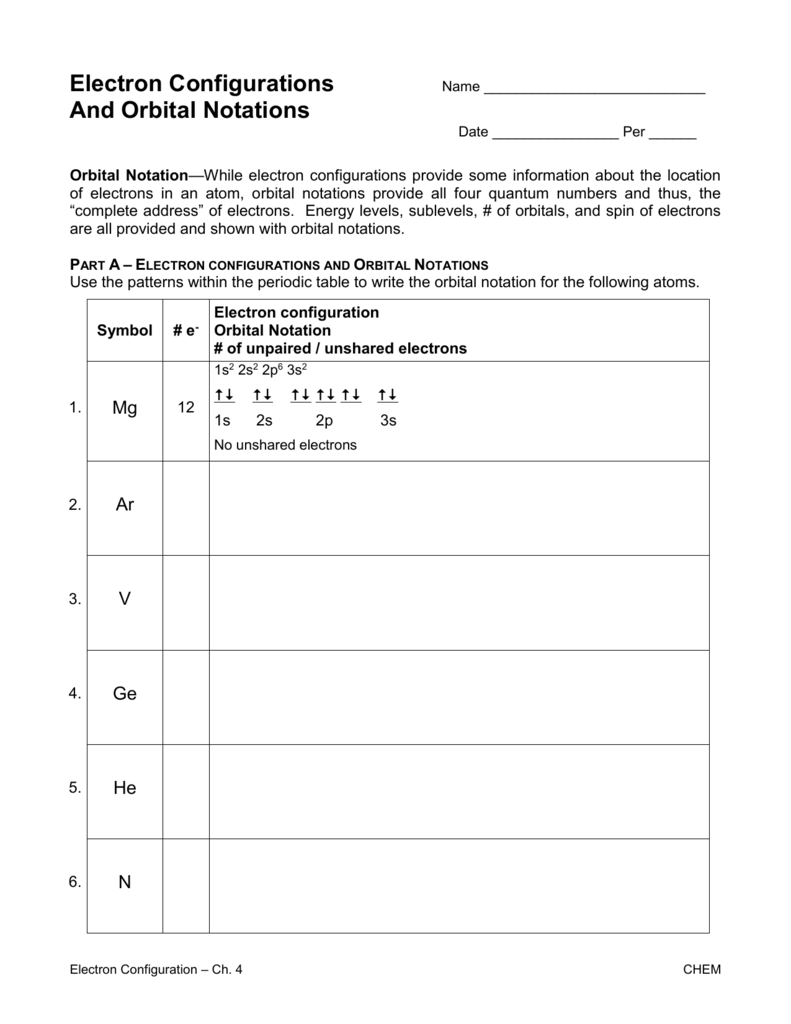
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen (H) | 1 | 1s¹ |
| Helium (He) | 2 | 1s² |
| Lithium (Li) | 3 | 1s², 2s¹ |
| Beryllium (Be) | 4 | 1s², 2s² |
| Boron (B) | 5 | 1s², 2s², 2p¹ |
| Carbon (C) | 6 | 1s², 2s², 2p² |
| Nitrogen (N) | 7 | 1s², 2s², 2p³ |
| Oxygen (O) | 8 | 1s², 2s², 2p⁴ |
| Fluorine (F) | 9 | 1s², 2s², 2p⁵ |
| Neon (Ne) | 10 | 1s², 2s², 2p⁶ |
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels.
How do I determine the electron configuration of an atom?
+Follow the 5 easy steps outlined in this article: Understand the basics of electron configuration, determine the atomic number, fill in the energy levels, use the periodic table to determine the electron configuration, and check your work.
What is the purpose of the periodic table in determining electron configuration?
+The periodic table helps us determine which energy levels are filled and which are not, allowing us to determine the electron configuration of an atom.
Related Terms:
- Electron configuration worksheet pdf
- Electron configuration pdf
- Electron configuration worksheet Doc
- Shorthand electron configuration worksheet answers