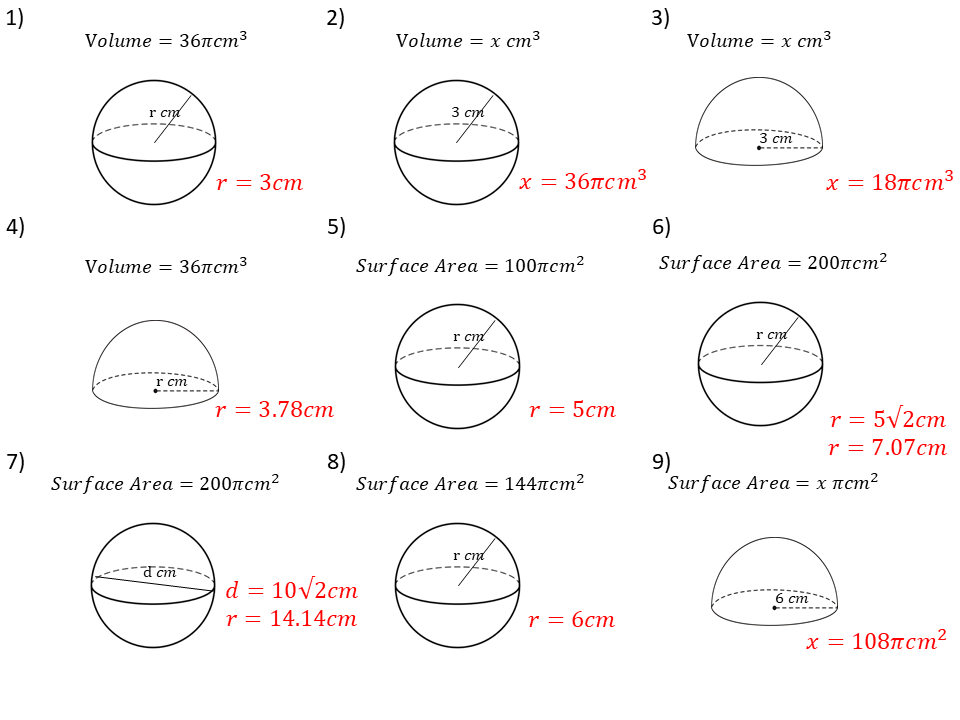Build an Atom Worksheet Answers Key

Atom Worksheet Answers Key
The atom is the basic building block of matter, and understanding its structure and properties is essential for chemistry and physics. Here is a worksheet answers key to help you assess your knowledge of atoms.
Section 1: Multiple Choice Questions
What is the smallest unit of matter that still retains the properties of an element?
- A. Molecule
- B. Compound
- C. Atom
- D. Mixture
Which of the following is a characteristic of an atom?
- A. Atoms are invisible to the naked eye.
- B. Atoms are made up of only protons.
- C. Atoms have a positive charge.
- D. Atoms are the building blocks of matter.
What is the atomic number of an element?
- A. The number of protons and neutrons in an atom’s nucleus.
- B. The number of electrons in an atom’s outermost energy level.
- C. The number of protons in an atom’s nucleus.
- D. The number of neutrons in an atom’s nucleus.
Section 2: Short Answer Questions
- Describe the structure of an atom.
Answer: An atom consists of a nucleus, which contains protons and neutrons, surrounded by electrons in energy levels or orbitals.
- What is the difference between an atom and a molecule?
Answer: An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of two or more atoms chemically bonded together.
Section 3: Essay Questions
- Explain the concept of atomic mass and how it differs from atomic number.
Answer: Atomic mass is the total number of protons and neutrons in an atom’s nucleus, while atomic number is the number of protons in an atom’s nucleus. Atomic mass is used to identify the mass of an atom, while atomic number is used to identify the element.
- Describe the different energy levels or orbitals in an atom and how electrons are arranged in them.
Answer: The different energy levels or orbitals in an atom are s, p, d, and f. Electrons are arranged in these orbitals according to the Aufbau principle and the Pauli Exclusion Principle. The s-orbital can hold up to 2 electrons, the p-orbital can hold up to 6 electrons, the d-orbital can hold up to 10 electrons, and the f-orbital can hold up to 14 electrons.
Section 4: Fill-in-the-Blank Questions
- The nucleus of an atom is made up of _______________________ and _______________________.
Answer: protons, neutrons
- The atomic number of an element is equal to the number of _______________________ in an atom’s nucleus.
Answer: protons
- The energy levels or orbitals in an atom are arranged according to the _______________________ principle.
Answer: Aufbau
Notes
📝 Note: The atomic number of an element is unique and identifies the element, while the atomic mass is not unique and can vary depending on the isotope.
📝 Note: The electrons in an atom are arranged in energy levels or orbitals, which are also known as electron shells.
FAQ Section
What is the smallest unit of matter that still retains the properties of an element?
+The smallest unit of matter that still retains the properties of an element is an atom.
What is the difference between an atom and a molecule?
+An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of two or more atoms chemically bonded together.
What is the atomic number of an element?
+The atomic number of an element is the number of protons in an atom's nucleus.
In conclusion, understanding the structure and properties of atoms is essential for chemistry and physics. This worksheet answers key provides a comprehensive review of the topic, covering multiple choice questions, short answer questions, essay questions, and fill-in-the-blank questions.
Related Terms:
- Build an atom PhET simulation
- PhET Build a molecule