6 Ways to Master Electron Configuration
Unlocking the Secrets of Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons in an atom. Mastering electron configuration is crucial for understanding various chemical properties and reactions. In this article, we will explore six ways to master electron configuration, making it easier for you to grasp this complex topic.
Understanding the Basics of Electron Configuration
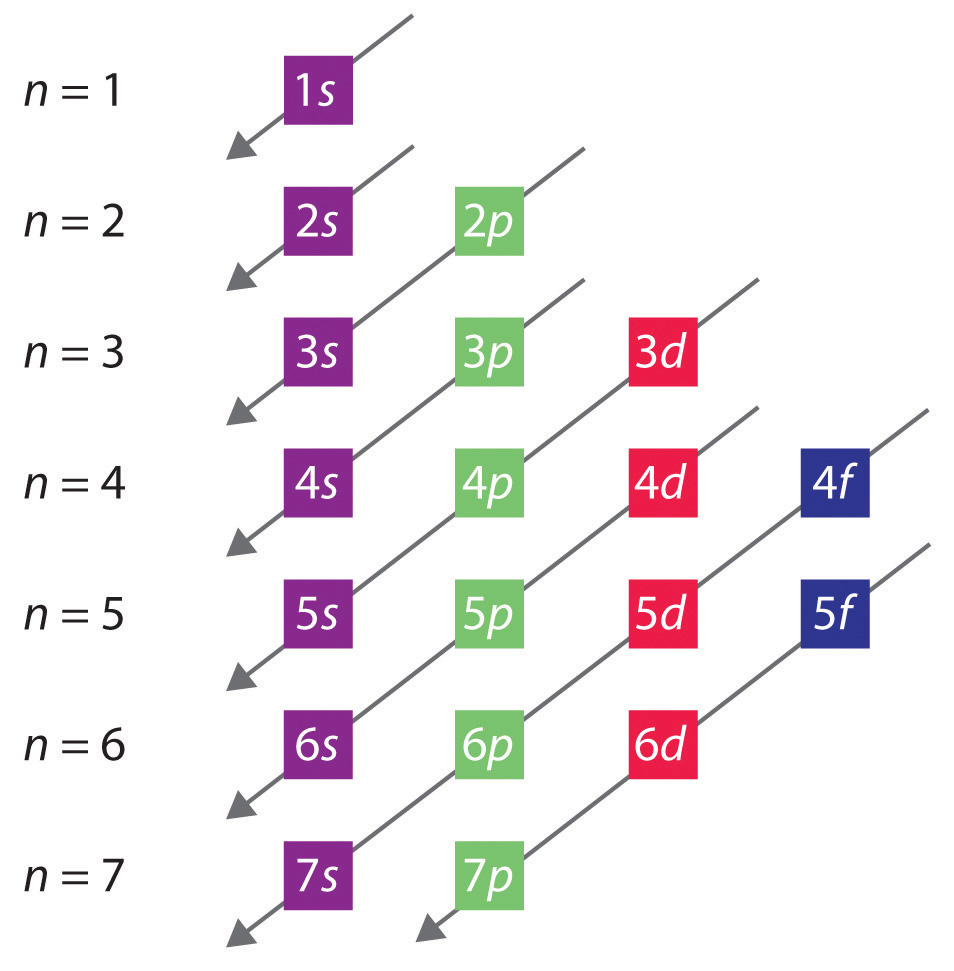
Before diving into the ways to master electron configuration, it’s essential to understand the basics. Electron configuration is a way of describing the arrangement of electrons in an atom, which is determined by the number of protons in the nucleus. The electrons occupy specific energy levels or shells, which are further divided into subshells. The subshells are filled in a specific order, following the Aufbau principle and the Pauli Exclusion Principle.
1. Learn the Aufbau Principle and the Pauli Exclusion Principle
The Aufbau principle states that electrons occupy the lowest available energy levels. The Pauli Exclusion Principle states that each orbital can hold a maximum of two electrons, which must have opposite spins. Understanding these principles is crucial for determining the electron configuration of an atom.
- Aufbau Principle: Electrons occupy the lowest available energy levels.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, which must have opposite spins.
2. Use the Periodic Table to Determine Electron Configuration
The periodic table is a powerful tool for determining electron configuration. By understanding the periodic trends and the block structure of the periodic table, you can quickly determine the electron configuration of an atom.
- Blocks of the Periodic Table: s-block, p-block, d-block, and f-block
- Periodic Trends: Atomic radius, electronegativity, and ionization energy
3. Practice Writing Electron Configurations
Practice is key to mastering electron configuration. Start by writing electron configurations for simple atoms, such as hydrogen and helium. Gradually move on to more complex atoms, such as carbon and oxygen.
- Example Electron Configurations:
- Hydrogen: 1s1
- Helium: 1s2
- Carbon: 1s2 2s2 2p2
- Oxygen: 1s2 2s2 2p4
4. Use Online Resources and Tools
There are many online resources and tools available to help you master electron configuration. Some popular resources include online periodic tables, electron configuration calculators, and interactive tutorials.
- Online Resources:
- Periodic tables
- Electron configuration calculators
- Interactive tutorials
5. Watch Video Tutorials and Lectures
Video tutorials and lectures can be a great way to learn electron configuration. Many online platforms, such as YouTube and Khan Academy, offer video tutorials and lectures on electron configuration.
- Video Tutorial Platforms:
- YouTube
- Khan Academy
- Crash Course
6. Take Practice Quizzes and Tests
Practice quizzes and tests can help you assess your understanding of electron configuration. Try taking online quizzes and tests to identify areas where you need improvement.
- Practice Quiz Platforms:
- Quizlet
- Kahoot
- Chemistry LibreTexts
📝 Note: Mastering electron configuration takes time and practice. Be patient and persistent, and you will see improvement over time.
In conclusion, mastering electron configuration requires a combination of understanding the basics, practicing writing electron configurations, using online resources and tools, watching video tutorials and lectures, and taking practice quizzes and tests. By following these six ways, you can become proficient in electron configuration and improve your understanding of chemistry.
What is electron configuration?
+Electron configuration is a way of describing the arrangement of electrons in an atom.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels.
How can I practice writing electron configurations?
+Start by writing electron configurations for simple atoms, such as hydrogen and helium. Gradually move on to more complex atoms, such as carbon and oxygen.