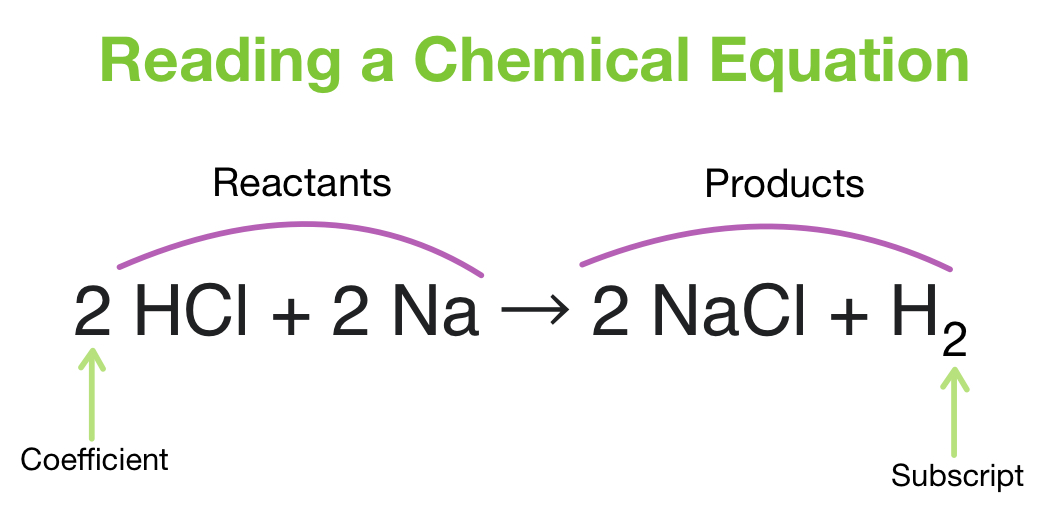
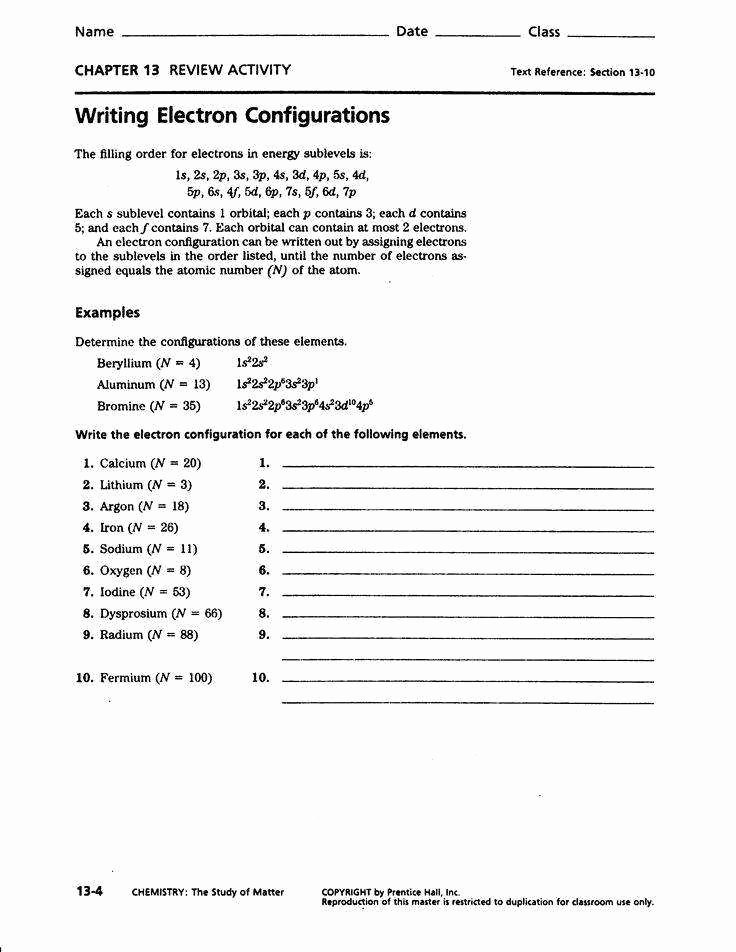
Electron Configuration Chem Worksheet 5-6

Understanding Electron Configuration
Electron configuration is a way of describing the arrangement of electrons in an atom. It is a fundamental concept in chemistry that helps us understand the properties and behavior of elements. In this worksheet, we will explore the basics of electron configuration and learn how to write electron configurations for atoms.
What is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom, which is determined by the number of electrons in the atom and the energy levels they occupy. Electrons occupy specific energy levels or shells around the nucleus of an atom. Each energy level has a limited number of electrons that it can hold.
How to Write Electron Configurations
To write an electron configuration, we need to follow a few simple steps:
- Determine the number of electrons in the atom.
- Identify the energy levels or shells that the electrons occupy.
- Write the electron configuration using the following notation:
- s (s orbital) can hold up to 2 electrons
- p (p orbital) can hold up to 6 electrons
- d (d orbital) can hold up to 10 electrons
- f (f orbital) can hold up to 14 electrons
For example, the electron configuration of sodium (Na) is 1s² 2s² 2p⁶ 3s¹.
Rules for Writing Electron Configurations
There are a few rules to keep in mind when writing electron configurations:
- Aufbau principle: Electrons occupy the lowest available energy levels first.
- Pauli exclusion principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s rule: Electrons occupy empty orbitals of the same energy level before pairing up in an orbital.
Examples of Electron Configurations
Here are a few examples of electron configurations:
- Hydrogen (H): 1s¹
- Helium (He): 1s²
- Lithium (Li): 1s² 2s¹
- Beryllium (Be): 1s² 2s²
- Boron (B): 1s² 2s² 2p¹
📝 Note: When writing electron configurations, make sure to follow the rules and notation above.
Electron Configuration and the Periodic Table
The periodic table is a powerful tool for understanding electron configuration. Elements in the same group (vertical column) have similar electron configurations, while elements in the same period (horizontal row) have the same number of electron shells.
| Element | Electron Configuration |
|---|---|
| Hydrogen (H) | 1s¹ |
| Helium (He) | 1s² |
| Lithium (Li) | 1s² 2s¹ |
| Beryllium (Be) | 1s² 2s² |
| Boron (B) | 1s² 2s² 2p¹ |
Conclusion
In conclusion, electron configuration is a fundamental concept in chemistry that helps us understand the properties and behavior of elements. By following the rules and notation above, we can write electron configurations for atoms and understand their relationships to the periodic table.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels first.
How many electrons can an s orbital hold?
+An s orbital can hold up to 2 electrons.
What is the electron configuration of sodium (Na)?
+The electron configuration of sodium (Na) is 1s² 2s² 2p⁶ 3s¹.
Related Terms:
- Electron configuration Worksheet answer Key
- Electron configuration PDF