5 Easy Ways to Balance Chemical Equations
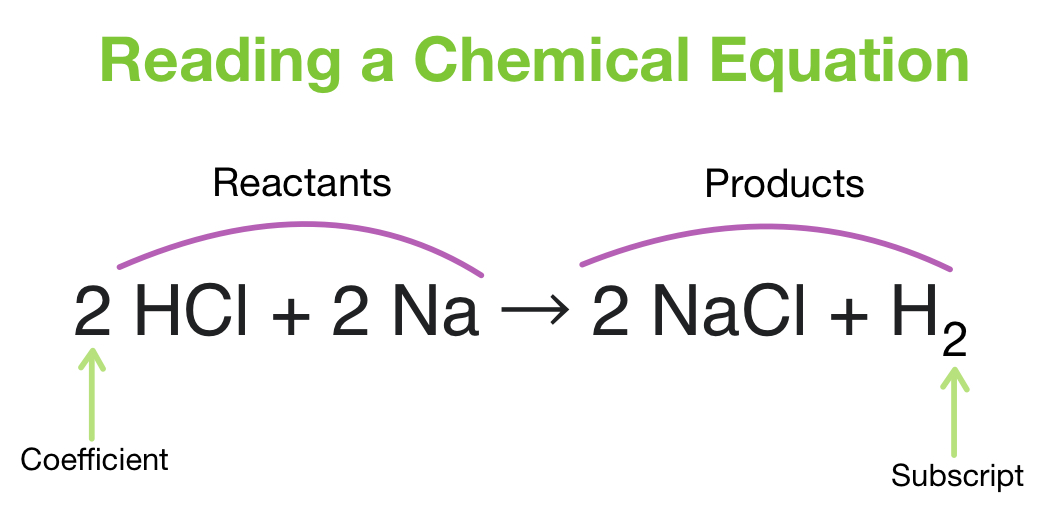
Introduction to Chemical Equations
Chemical equations are a crucial part of chemistry, representing the reaction between substances. However, to accurately depict these reactions, equations must be balanced. A balanced equation ensures that the number of atoms for each element is the same on both the reactant and product sides. In this article, we will explore five easy ways to balance chemical equations.
Understanding the Importance of Balancing Chemical Equations
Before diving into the methods, it’s essential to understand why balancing chemical equations is crucial:
- Conservation of Mass: Chemical reactions involve the transformation of substances, but the total mass remains constant. A balanced equation ensures that the number of atoms for each element is conserved.
- Accuracy: Unbalanced equations can lead to incorrect conclusions about the reaction, including the amount of reactants and products.
- Safety: In industrial settings, unbalanced equations can lead to safety hazards, such as the production of unwanted byproducts.
Method 1: Trial and Error
One of the simplest methods for balancing chemical equations is trial and error. This involves:
- Writing the Unbalanced Equation: Start by writing the unbalanced equation with the reactants on the left and products on the right.
- Counting Atoms: Count the number of atoms for each element on both sides of the equation.
- Adjusting Coefficients: Adjust the coefficients (numbers in front of the formulas of reactants or products) to balance the equation.
👀 Note: This method can be time-consuming and may not always result in the simplest solution.
Method 2: The Balancing Algorithm
The balancing algorithm is a step-by-step approach to balancing chemical equations:
- Write the Unbalanced Equation: Write the unbalanced equation with the reactants on the left and products on the right.
- Identify the Elements: Identify the elements that appear on both sides of the equation.
- Count Atoms: Count the number of atoms for each element on both sides of the equation.
- Balance Elements: Balance each element one at a time, starting with elements that appear only once on each side.
- Check the Equation: Check the equation to ensure that it is balanced.
📝 Note: This method can be more efficient than trial and error, but it still requires careful attention to detail.
Method 3: The Half-Reaction Method
The half-reaction method involves:
- Breaking the Equation: Breaking the equation into two half-reactions: one for oxidation and one for reduction.
- Balancing Half-Reactions: Balancing each half-reaction separately.
- Combining Half-Reactions: Combining the balanced half-reactions to form the final equation.
🔍 Note: This method is particularly useful for redox reactions, where it can be challenging to balance the equation using other methods.
Method 4: Using Matrices
Using matrices is a more advanced method for balancing chemical equations:
- Creating a Matrix: Creating a matrix with the coefficients of the elements as rows and the reactants and products as columns.
- Solving the Matrix: Solving the matrix to find the values of the coefficients that balance the equation.
📊 Note: This method requires a good understanding of linear algebra and can be time-consuming to set up.
Method 5: Online Tools
There are many online tools available that can balance chemical equations:
- Entering the Equation: Entering the unbalanced equation into the tool.
- Generating the Balanced Equation: The tool generates the balanced equation.
👍 Note: This method is quick and easy, but it's essential to understand the underlying chemistry and not just rely on the tool.
Conclusion
Balancing chemical equations is a crucial step in understanding chemical reactions. By using one of the five methods outlined above, you can ensure that your equations are accurate and reliable. Whether you prefer the simplicity of trial and error or the efficiency of online tools, there is a method to suit your needs.
What is the importance of balancing chemical equations?
+
Balancing chemical equations is crucial for ensuring the conservation of mass, accuracy, and safety in chemical reactions.
What are the five methods for balancing chemical equations?
+
The five methods are trial and error, the balancing algorithm, the half-reaction method, using matrices, and online tools.
Which method is most efficient for balancing chemical equations?
+
The most efficient method depends on the complexity of the equation and personal preference. However, online tools can be a quick and easy solution.