5 Ways to Master Counting Atoms Worksheet Answers
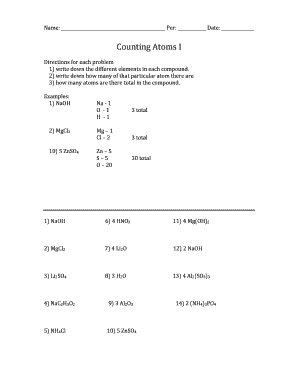
Mastering Counting Atoms Worksheet Answers: A Comprehensive Guide
Counting atoms is a fundamental concept in chemistry that requires a strong understanding of atomic structure and chemical formulas. As a student, you may have encountered difficulties in solving counting atoms worksheet problems. In this article, we will provide you with a comprehensive guide on how to master counting atoms worksheet answers. We will cover the five ways to help you become proficient in solving these problems.
Understanding Atomic Structure
Before diving into counting atoms, it’s essential to understand the basic structure of an atom. An atom consists of three main parts: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons orbit around the nucleus. The number of protons in an atom determines the element of an atom, and each element has a unique atomic number.
Key Points to Remember:
- Protons are positively charged particles found in the nucleus.
- Neutrons are neutral particles found in the nucleus.
- Electrons are negatively charged particles that orbit around the nucleus.
- The number of protons in an atom determines the element of an atom.
Way 1: Identify the Chemical Formula
To solve counting atoms problems, you need to identify the chemical formula of the compound. A chemical formula represents the number of atoms of each element in a compound. For example, the chemical formula for water is H2O, which means there are two hydrogen atoms and one oxygen atom in a molecule of water.
Tips for Identifying Chemical Formulas:
- Look for the chemical formula in the problem statement.
- Use the periodic table to determine the number of protons in each element.
- Determine the number of atoms of each element in the compound.
Way 2: Count Atoms in Simple Compounds
Once you have identified the chemical formula, you can count the number of atoms in simple compounds. Simple compounds are compounds that consist of only two elements, such as water (H2O) or carbon dioxide (CO2).
Steps to Count Atoms in Simple Compounds:
- Identify the chemical formula of the compound.
- Determine the number of atoms of each element in the compound.
- Count the total number of atoms in the compound.
📝 Note: Make sure to count the atoms of each element correctly. For example, in the compound H2O, there are two hydrogen atoms and one oxygen atom.
Way 3: Count Atoms in Complex Compounds
Complex compounds are compounds that consist of more than two elements, such as ammonia (NH3) or glucose (C6H12O6). To count atoms in complex compounds, you need to follow these steps:
Steps to Count Atoms in Complex Compounds:
- Identify the chemical formula of the compound.
- Determine the number of atoms of each element in the compound.
- Count the total number of atoms in the compound.
- Use parentheses to group atoms together if necessary.
📝 Note: Make sure to use parentheses correctly when counting atoms in complex compounds. For example, in the compound C6H12O6, the parentheses are not necessary.
Way 4: Count Atoms in Ionic Compounds
Ionic compounds are compounds that consist of ions, which are atoms or groups of atoms that have gained or lost electrons. To count atoms in ionic compounds, you need to follow these steps:
Steps to Count Atoms in Ionic Compounds:
- Identify the chemical formula of the compound.
- Determine the number of ions in the compound.
- Count the total number of atoms in the compound.
📝 Note: Make sure to count the ions correctly. For example, in the compound NaCl, there is one sodium ion and one chloride ion.
Way 5: Practice, Practice, Practice
The final way to master counting atoms worksheet answers is to practice, practice, practice. The more you practice, the more comfortable you will become with counting atoms. Try solving different types of problems, such as simple compounds, complex compounds, and ionic compounds.
Tips for Practicing:
- Start with simple compounds and gradually move on to more complex compounds.
- Use online resources, such as worksheets and quizzes, to practice counting atoms.
- Review the concepts regularly to reinforce your understanding.
Mastering counting atoms worksheet answers requires practice and a strong understanding of atomic structure and chemical formulas. By following these five ways, you can become proficient in solving counting atoms problems and excel in your chemistry studies.
In conclusion, mastering counting atoms worksheet answers is a skill that can be developed with practice and a strong understanding of atomic structure and chemical formulas. By identifying the chemical formula, counting atoms in simple and complex compounds, counting atoms in ionic compounds, and practicing regularly, you can become proficient in solving counting atoms problems.
What is the importance of understanding atomic structure in counting atoms?
+
Understanding atomic structure is crucial in counting atoms because it helps you determine the number of protons in an atom, which in turn determines the element of an atom.
How do I count atoms in complex compounds?
+
To count atoms in complex compounds, you need to identify the chemical formula, determine the number of atoms of each element, count the total number of atoms, and use parentheses to group atoms together if necessary.
What is the difference between simple and complex compounds?
+
Simple compounds are compounds that consist of only two elements, while complex compounds are compounds that consist of more than two elements.