5 Ways to Master Counting Atoms

Mastering the art of counting atoms is a fundamental skill for any chemistry enthusiast. It may seem daunting at first, but with practice and the right strategies, you can become a pro at counting atoms in no time. In this article, we will explore five ways to help you master counting atoms.
Understanding the Basics
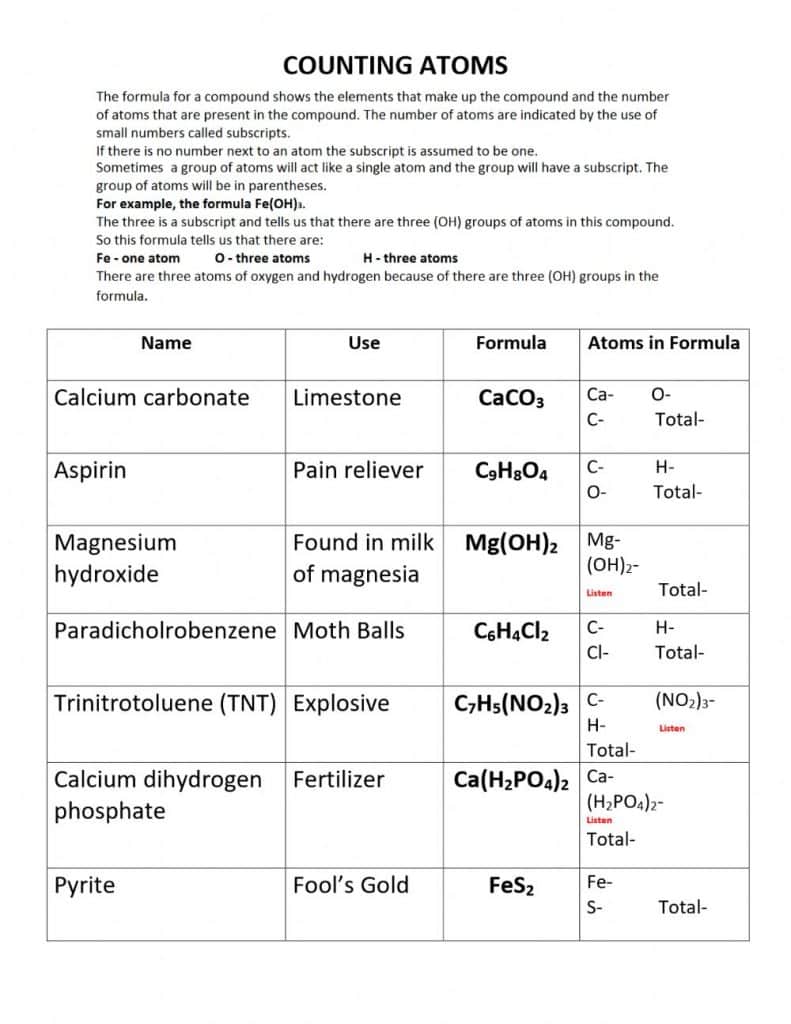
Before we dive into the five ways to master counting atoms, it’s essential to understand the basics. Counting atoms involves determining the number of atoms of each element present in a molecule or compound. This can be done using various formulas and techniques, which we will discuss later.
1. Start with Simple Compounds
One of the best ways to master counting atoms is to start with simple compounds. Begin with basic compounds like water (H2O) or carbon dioxide (CO2). These compounds have a limited number of atoms, making it easier to count them. As you become more comfortable, you can move on to more complex compounds.
📝 Note: When counting atoms, always start with the simplest compounds and gradually move to more complex ones.
2. Use the Periodic Table
The periodic table is an essential tool for counting atoms. It provides valuable information about each element, including its atomic number, mass number, and valency. By using the periodic table, you can determine the number of atoms of each element in a compound.
| Element | Atomic Number | Mass Number | Valency |
|---|---|---|---|
| Hydrogen | 1 | 1 | 1 |
| Carbon | 6 | 12 | 4 |
| Oxygen | 8 | 16 | 2 |
3. Apply the Rules of Valency
Valency is the number of electrons an atom can gain or lose to form a bond. Understanding valency is crucial for counting atoms. By applying the rules of valency, you can determine the number of atoms of each element in a compound.
- Metals typically have a positive valency, while non-metals have a negative valency.
- The valency of an element determines the number of atoms it can bond with.
- The sum of valencies of all atoms in a compound must be zero.
4. Use Formulas and Equations
Formulas and equations are essential tools for counting atoms. By using formulas like the molecular formula or the empirical formula, you can determine the number of atoms of each element in a compound.
- Molecular formula: The molecular formula shows the actual number of atoms of each element in a molecule.
- Empirical formula: The empirical formula shows the simplest whole-number ratio of atoms of each element in a compound.
5. Practice, Practice, Practice
Finally, the key to mastering counting atoms is practice. The more you practice, the more comfortable you will become with counting atoms. Start with simple compounds and gradually move on to more complex ones.
📝 Note: Practice is essential for mastering counting atoms. The more you practice, the more accurate you will become.
Mastering counting atoms is a skill that takes time and practice to develop. By following these five ways, you can become a pro at counting atoms in no time. Remember to start with simple compounds, use the periodic table, apply the rules of valency, use formulas and equations, and practice regularly.
In conclusion, counting atoms is an essential skill for any chemistry enthusiast. By mastering this skill, you can improve your understanding of chemistry and become more proficient in solving chemistry problems.
What is the difference between a molecular formula and an empirical formula?
+
A molecular formula shows the actual number of atoms of each element in a molecule, while an empirical formula shows the simplest whole-number ratio of atoms of each element in a compound.
How do I use the periodic table to count atoms?
+
By using the periodic table, you can determine the number of atoms of each element in a compound. Look up the element on the periodic table and note its atomic number, mass number, and valency.
What is valency and how does it relate to counting atoms?
+
Valency is the number of electrons an atom can gain or lose to form a bond. Understanding valency is crucial for counting atoms, as it determines the number of atoms of each element in a compound.