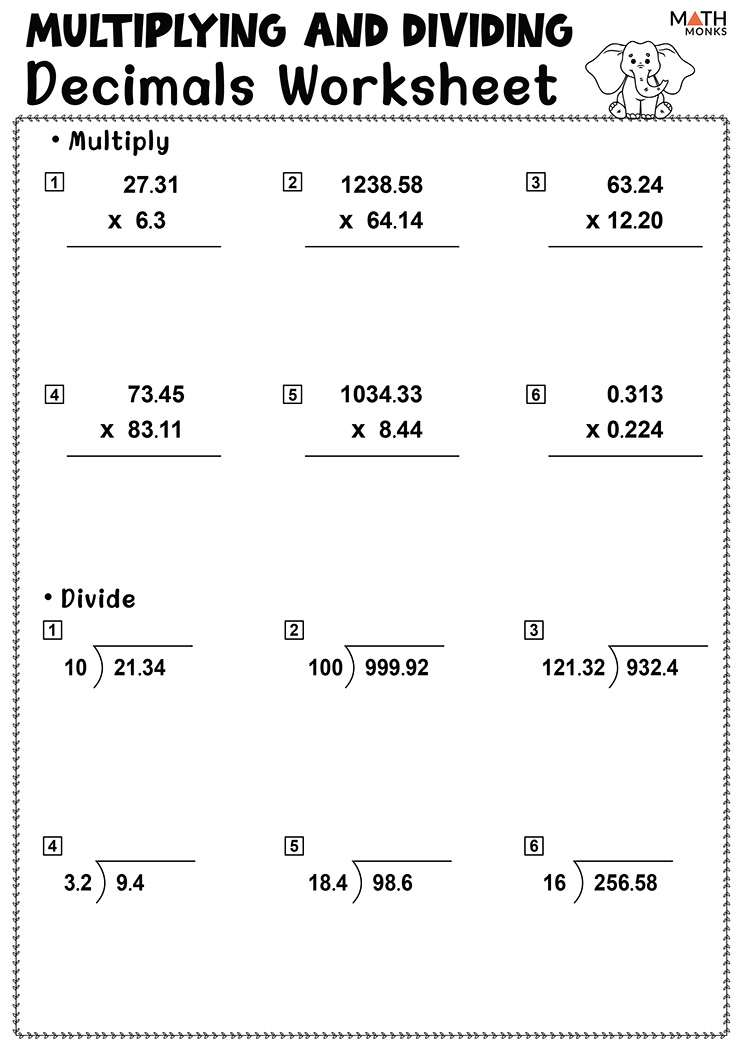
Combined Gas Law Worksheet Answers Guide

Understanding the Combined Gas Law
The combined gas law is a fundamental concept in chemistry that describes the relationship between pressure, volume, and temperature of a gas. It is a combination of Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law, which are all special cases of the ideal gas law. In this article, we will provide a comprehensive guide to the combined gas law, including its definition, formula, and examples.
Definition and Formula
The combined gas law states that, at constant temperature, the volume of a gas is inversely proportional to the pressure, and at constant pressure, the volume of a gas is directly proportional to the temperature. Mathematically, this is expressed as:
P1V1/T1 = P2V2/T2
Where:
- P1 and P2 are the initial and final pressures of the gas
- V1 and V2 are the initial and final volumes of the gas
- T1 and T2 are the initial and final temperatures of the gas in Kelvin
How to Use the Combined Gas Law
To use the combined gas law, you need to know the initial and final conditions of the gas, including pressure, volume, and temperature. Here are the steps to follow:
- Identify the given values: Write down the given values of P1, V1, T1, and the unknown values you need to find.
- Rearrange the equation: Rearrange the combined gas law equation to solve for the unknown value.
- Plug in the values: Substitute the given values into the rearranged equation.
- Solve for the unknown: Solve for the unknown value.
Examples and Solutions
Here are some examples to illustrate the use of the combined gas law:
Example 1
A gas cylinder contains 2.0 L of oxygen at a pressure of 150 kPa and a temperature of 25°C. If the pressure is increased to 200 kPa while the temperature remains constant, what is the new volume of the gas?
Solution
Given values: P1 = 150 kPa, V1 = 2.0 L, T1 = 25°C = 298 K, P2 = 200 kPa Unknown value: V2
Rearranged equation: V2 = V1 × (P1/P2) × (T2/T1)
Plug in values: V2 = 2.0 L × (150 kPa/200 kPa) × (298 K/298 K) Solve for V2: V2 = 1.5 L
Example 2
A gas sample is collected at a pressure of 1.0 atm and a temperature of 30°C. If the temperature is increased to 50°C while the pressure remains constant, what is the new volume of the gas?
Solution
Given values: P1 = 1.0 atm, T1 = 30°C = 303 K, T2 = 50°C = 323 K Unknown values: V2 (assuming V1 is constant)
Rearranged equation: V2 = V1 × (T2/T1)
Plug in values: V2 = V1 × (323 K/303 K) Solve for V2: V2 = 1.06 V1
Example 3
A gas is compressed from an initial volume of 5.0 L to a final volume of 2.0 L at a constant temperature of 20°C. If the initial pressure was 100 kPa, what is the final pressure of the gas?
Solution
Given values: V1 = 5.0 L, V2 = 2.0 L, T1 = 20°C = 293 K, P1 = 100 kPa Unknown value: P2
Rearranged equation: P2 = P1 × (V1/V2) × (T2/T1)
Plug in values: P2 = 100 kPa × (5.0 L/2.0 L) × (293 K/293 K) Solve for P2: P2 = 250 kPa
Important Notes
- Always use Kelvin temperatures when applying the combined gas law.
- Be careful when rearranging the equation to solve for the unknown value.
- Use dimensional analysis to ensure that the units are correct.
💡 Note: The combined gas law assumes ideal gas behavior, which may not be true for all real gases.
In conclusion, the combined gas law is a powerful tool for understanding the behavior of gases under different conditions. By following the steps outlined above and using the formula correctly, you can solve a wide range of problems involving pressure, volume, and temperature.
What is the combined gas law?
+The combined gas law is a fundamental concept in chemistry that describes the relationship between pressure, volume, and temperature of a gas.
How do I use the combined gas law?
+To use the combined gas law, identify the given values, rearrange the equation to solve for the unknown value, plug in the values, and solve for the unknown.
What are the limitations of the combined gas law?
+The combined gas law assumes ideal gas behavior, which may not be true for all real gases.