5 Color Coding Hacks for the Periodic Table
The periodic table is a powerful tool for chemists and chemistry students, but it can be overwhelming to navigate, especially for beginners. One way to make the periodic table more manageable is to use color coding to highlight important trends and patterns. Here are five color coding hacks to help you master the periodic table:
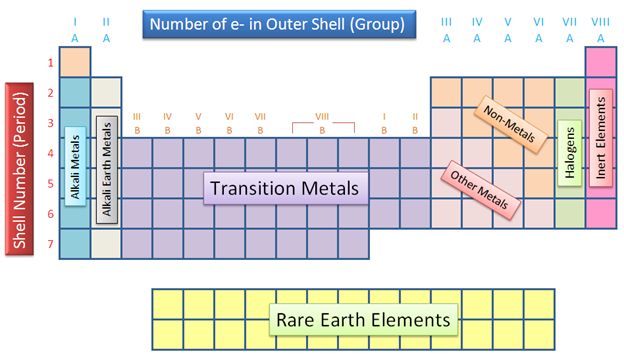
1. Metals, Nonmetals, and Metalloids
One of the most basic and useful color coding hacks is to distinguish between metals, nonmetals, and metalloids. You can use three different colors to highlight these categories:
- Metals: Use a shade of blue to highlight the metals, which are typically found on the left side and center of the periodic table.
- Nonmetals: Use a shade of red to highlight the nonmetals, which are typically found on the right side of the periodic table.
- Metalloids: Use a shade of green to highlight the metalloids, which are found on the border between the metals and nonmetals.
This color coding system will help you quickly identify the properties of an element based on its position on the periodic table.
2. Periodic Trends
The periodic table exhibits several trends that can be highlighted using color coding. Here are a few examples:
- Atomic Radius: Use a gradient of colors to show how atomic radius decreases from left to right across a period.
- Electronegativity: Use a gradient of colors to show how electronegativity increases from left to right across a period.
- Ionization Energy: Use a gradient of colors to show how ionization energy increases from left to right across a period.
By highlighting these trends, you’ll be able to see how the properties of elements change as you move across a period or down a group.
3. Group and Period Numbers
Another useful color coding hack is to highlight the group and period numbers of each element. You can use different colors to distinguish between the different groups and periods:
- Group Numbers: Use a different color for each group number (e.g., Group 1 in red, Group 2 in blue, etc.).
- Period Numbers: Use a different color for each period number (e.g., Period 1 in green, Period 2 in yellow, etc.).
This will help you quickly identify the group and period numbers of an element, which is essential for understanding its properties and behavior.
4. Block Elements
The periodic table can also be divided into blocks based on the orbital type of the elements. You can use color coding to highlight these blocks:
- s-Block: Use a shade of yellow to highlight the s-block elements, which are found in Groups 1 and 2.
- p-Block: Use a shade of orange to highlight the p-block elements, which are found in Groups 13-18.
- d-Block: Use a shade of purple to highlight the d-block elements, which are found in the transition metals.
- f-Block: Use a shade of pink to highlight the f-block elements, which are found in the inner transition metals.
By highlighting these blocks, you’ll be able to see how the orbital type of an element affects its properties and behavior.
5. Radioactive Elements
Finally, you can use color coding to highlight the radioactive elements, which are typically found on the right side of the periodic table. Use a shade of gray or black to highlight these elements, which will help you quickly identify them.
💡 Note: Radioactive elements are not necessarily "bad" or "dangerous," but rather, they have unstable nuclei that undergo radioactive decay.
By using these five color coding hacks, you’ll be able to navigate the periodic table with confidence and quickly identify the properties and trends of different elements.
The periodic table is a powerful tool for chemists and chemistry students, but it can be overwhelming to navigate, especially for beginners. By using color coding to highlight important trends and patterns, you can make the periodic table more manageable and increase your understanding of chemistry.
In conclusion, mastering the periodic table requires a combination of knowledge, practice, and visual aids. By using color coding hacks to highlight important trends and patterns, you can unlock the secrets of the periodic table and become a more confident and proficient chemist.
What is the periodic table?
+The periodic table is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties.
Why is color coding useful for the periodic table?
+Color coding is useful for the periodic table because it helps to highlight important trends and patterns, making it easier to navigate and understand the properties and behavior of different elements.
What are some common color coding systems for the periodic table?
+Some common color coding systems for the periodic table include distinguishing between metals, nonmetals, and metalloids, highlighting periodic trends, and identifying block elements.
Related Terms:
- Tabel Periodik
- Blok-f
- Hidrogen
- Reaksi kimia
- Ikatan kimia
- Kimia