Chemistry Metric Conversions Made Easy with Practice
Chemistry Metric Conversions Made Easy with Practice
Metric conversions are an essential part of chemistry, and mastering them can make a significant difference in your understanding and application of chemical concepts. In this article, we will explore the world of metric conversions, providing you with a comprehensive guide on how to perform them with ease.
Why Are Metric Conversions Important in Chemistry?
Metric conversions are crucial in chemistry because they enable you to express quantities in a standardized unit system. The metric system, also known as the International System of Units (SI), is used universally in scientific and technical applications. By converting between different units, you can:
- Express measurements in a consistent and reproducible manner
- Compare and contrast data from different sources
- Perform calculations with accuracy and precision
- Communicate effectively with other scientists and professionals
Basic Concepts and Terminology
Before diving into metric conversions, let’s review some basic concepts and terminology:
- Unit: A standard quantity used to express a physical quantity (e.g., meter, gram, liter)
- Conversion factor: A ratio of two units used to convert between them (e.g., 1 meter = 100 centimeters)
- Prefix: A symbol used to denote a multiple or fraction of a unit (e.g., kilo-, centi-, milli-)
- Base unit: A fundamental unit that cannot be expressed in terms of other units (e.g., meter, gram, second)
Common Metric Conversion Factors
Here are some common metric conversion factors to get you started:
- Length:
- 1 meter (m) = 100 centimeters (cm) = 1000 millimeters (mm)
- 1 kilometer (km) = 1000 meters (m)
- Mass:
- 1 gram (g) = 1000 milligrams (mg) = 1000000 micrograms (μg)
- 1 kilogram (kg) = 1000 grams (g)
- Volume:
- 1 liter (L) = 1000 milliliters (mL) = 1000000 microliters (μL)
- 1 milliliter (mL) = 1 cubic centimeter (cm³)
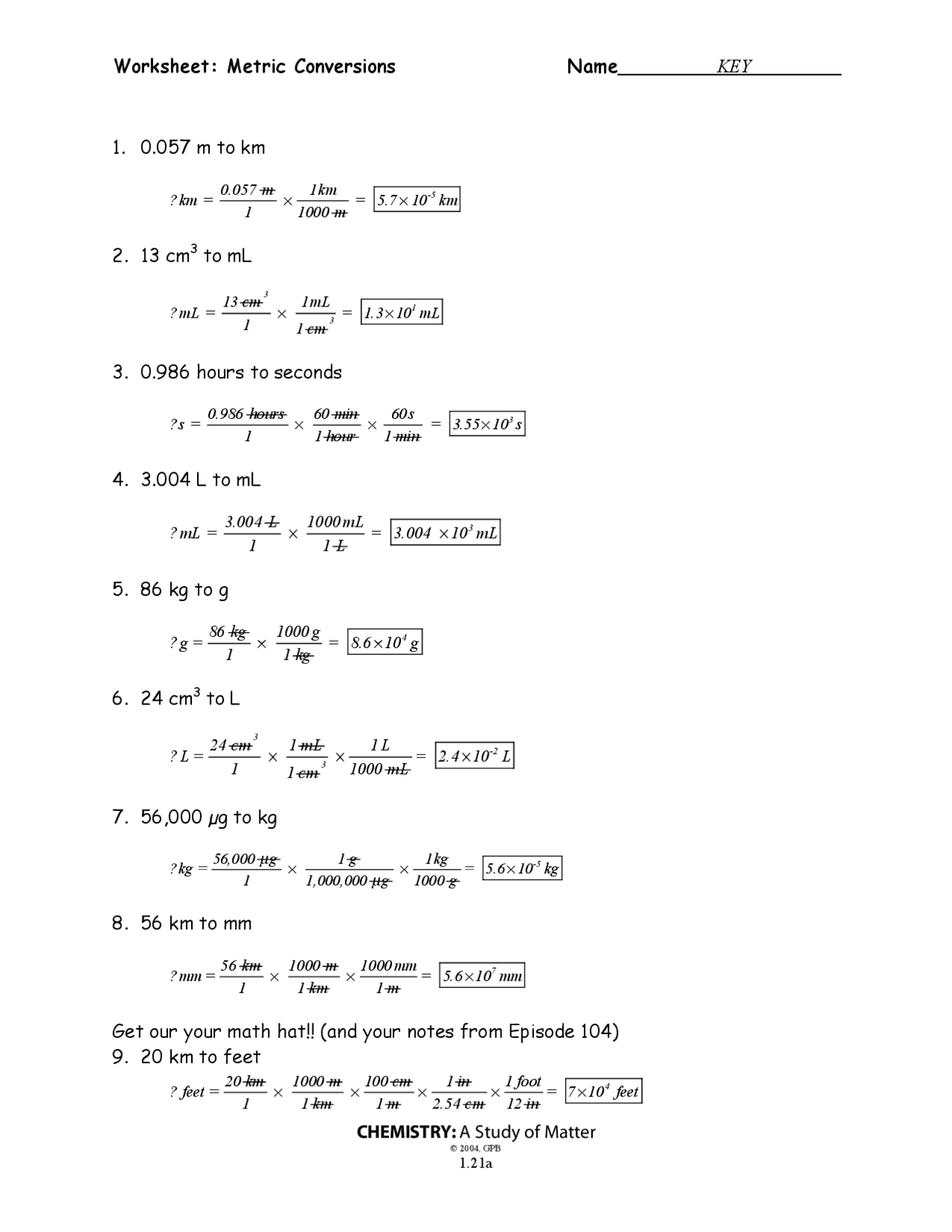
Converting Between Units
To convert between units, follow these steps:
- Identify the given unit and the desired unit.
- Find the conversion factor between the two units.
- Multiply the given value by the conversion factor.
For example, let’s convert 500 milliliters to liters:
- Given unit: milliliters (mL)
- Desired unit: liters (L)
- Conversion factor: 1 L = 1000 mL
- Calculation: 500 mL × (1 L / 1000 mL) = 0.5 L
Practice Problems and Solutions
Practice makes perfect! Here are some practice problems to help you master metric conversions:
- Convert 250 grams to kilograms.
- Solution: 250 g × (1 kg / 1000 g) = 0.25 kg
- Convert 3.5 liters to milliliters.
- Solution: 3.5 L × (1000 mL / 1 L) = 3500 mL
- Convert 450 centimeters to meters.
- Solution: 450 cm × (1 m / 100 cm) = 4.5 m
🔍 Note: When converting between units, make sure to check the units of the conversion factor to ensure accuracy.
Common Mistakes to Avoid
When performing metric conversions, avoid these common mistakes:
- Inconsistent units: Make sure to use consistent units throughout your calculations.
- Incorrect conversion factors: Double-check your conversion factors to avoid errors.
- Rounding errors: Avoid rounding too early in your calculations, as this can lead to significant errors.
Conclusion
Mastering metric conversions is a crucial skill for any chemistry student or professional. By understanding the basics of metric conversions, practicing with common conversion factors, and avoiding common mistakes, you can become proficient in performing metric conversions with ease.
What is the most common unit of measurement in chemistry?
+The most common unit of measurement in chemistry is the metric system, specifically the International System of Units (SI).
How do I convert between units in the metric system?
+To convert between units, identify the given unit and the desired unit, find the conversion factor between the two units, and multiply the given value by the conversion factor.
What are some common mistakes to avoid when performing metric conversions?
+Common mistakes to avoid include inconsistent units, incorrect conversion factors, and rounding errors.
Related Terms:
- Unit conversions Worksheet
- Unit conversion Worksheet PDF
- Unit conversions Worksheet with answers