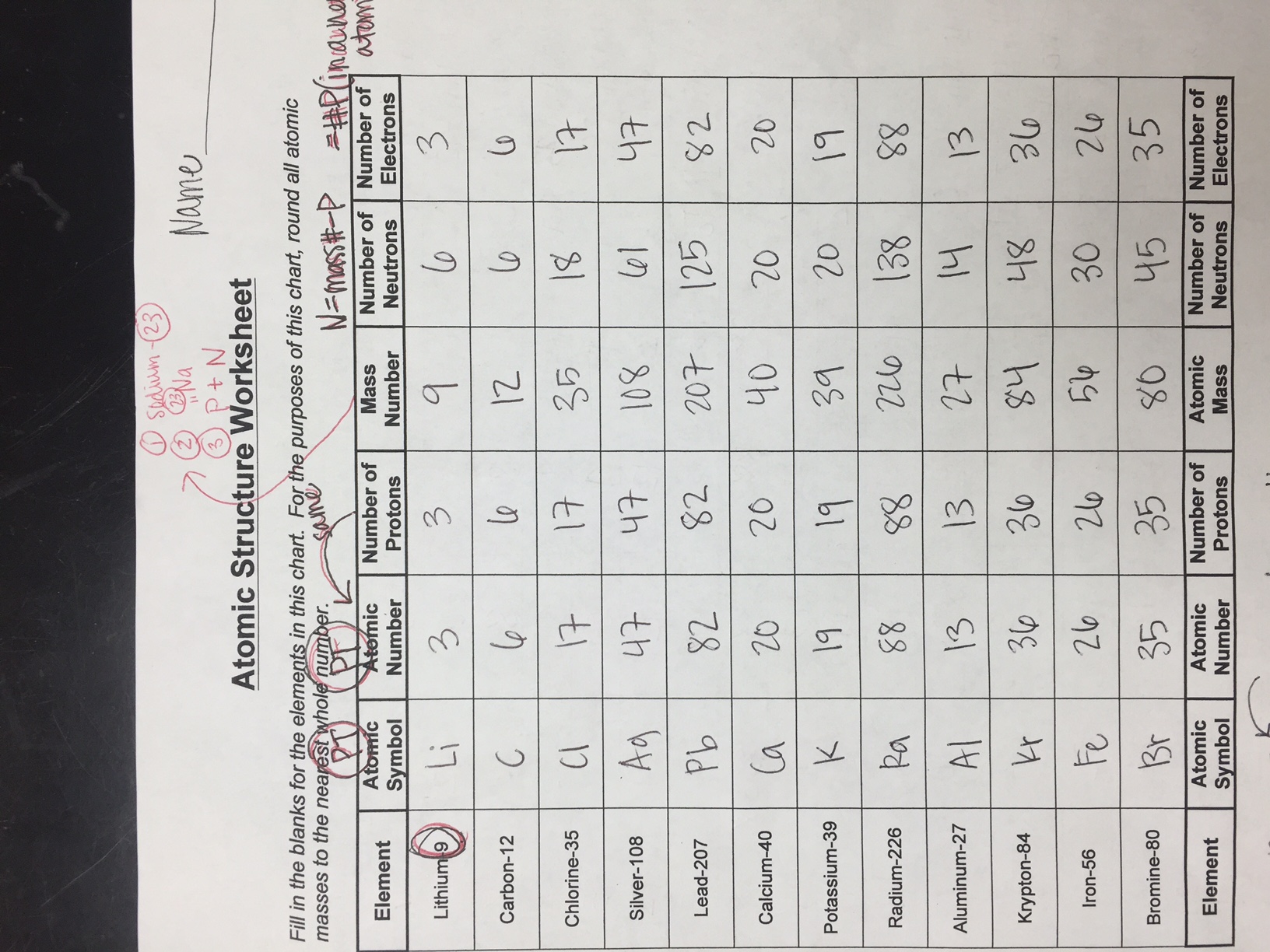
Atomic Structure Worksheet Answer Key for Chemistry Students
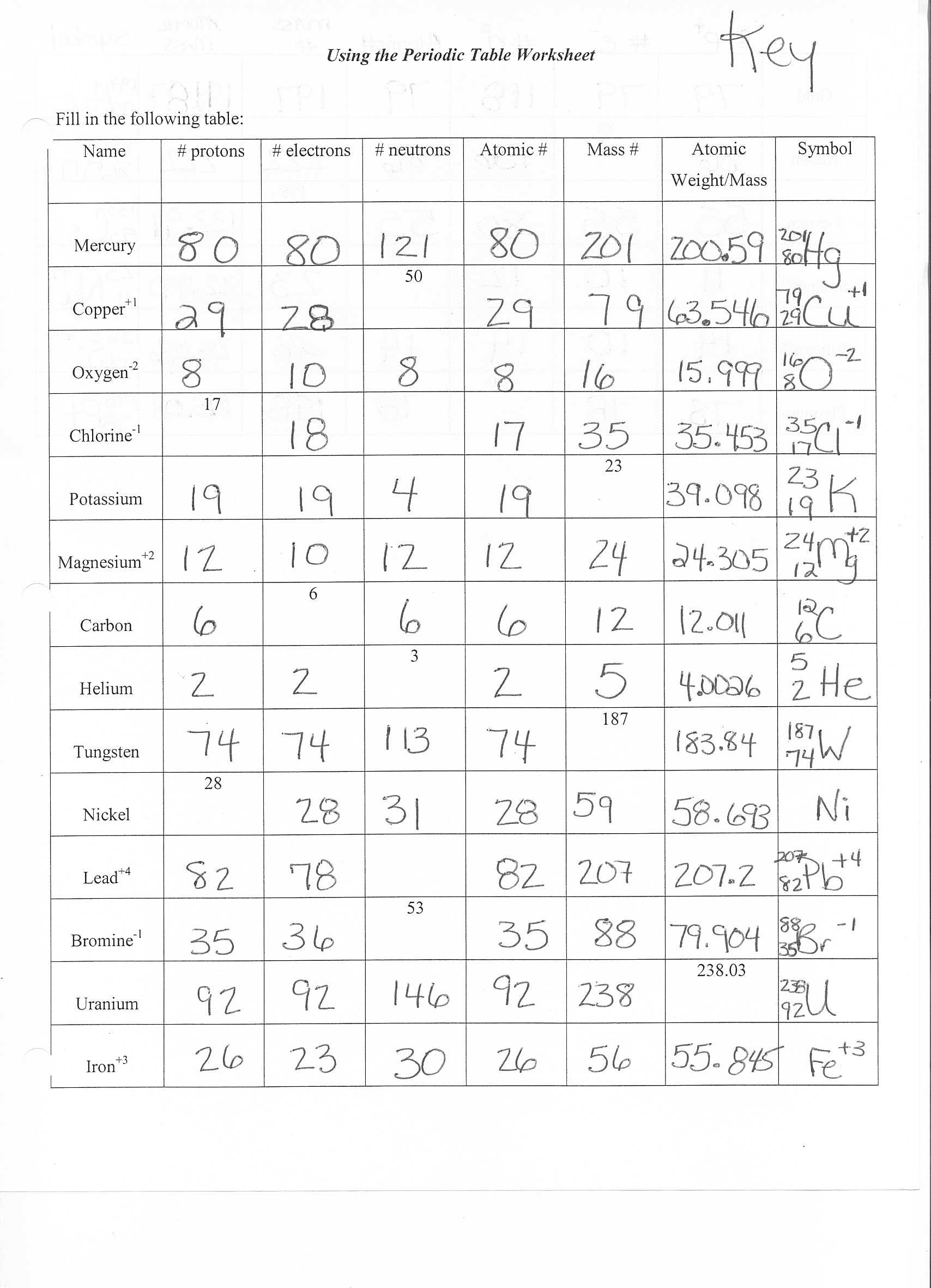
Understanding the Building Blocks of Matter: Atomic Structure Worksheet Answer Key
As chemistry students, understanding the atomic structure is crucial for grasping the fundamental principles of chemistry. The atomic structure worksheet is designed to help students learn and practice the concepts of atomic structure, including the components of an atom, electron configuration, and more. Here’s the answer key to the atomic structure worksheet:
Section 1: Multiple Choice Questions
What is the smallest unit of matter that still retains the properties of an element?
- Molecule
- Compound
- Atom
- Mixture
Which of the following is NOT a subatomic particle?
- Proton
- Neutron
- Electron
- Molecule
What is the charge of a proton?
- Positive
- Negative
- Neutral
- Zero
Section 2: Short Answer Questions
- What is the atomic number of an element, and how is it related to the number of protons in an atom?
Answer: The atomic number of an element is the number of protons present in the nucleus of an atom. It is a unique identifier for each element and determines the element’s position in the periodic table.
- Describe the difference between an electron shell and an energy level.
Answer: An electron shell refers to the region around the nucleus where electrons are found, while an energy level refers to the specific energy state that an electron occupies within a shell.
Section 3: Fill-in-the-Blank Questions
- The atomic mass of an element is the total number of _______________________ and _______________________ in an atom.
Answer: protons and neutrons
- The electron configuration of an atom is a way of describing the _______________________ of electrons in an atom.
Answer: arrangement
Section 4: Essay Question
- Describe the structure of an atom, including the components of the nucleus and the electron cloud. Be sure to include the relationships between protons, neutrons, and electrons.
Answer:
The atom is the smallest unit of matter that still retains the properties of an element. At the center of the atom is the nucleus, which contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. The number of protons in an atom determines the element’s atomic number and its position in the periodic table.
Surrounding the nucleus is the electron cloud, which is composed of electrons that occupy specific energy levels or shells. The electrons in an atom are arranged in a specific pattern, with each energy level able to hold a specific number of electrons. The electron configuration of an atom is a way of describing the arrangement of electrons in an atom.
The relationship between protons, neutrons, and electrons is crucial to understanding the structure of an atom. The number of protons in an atom determines the number of electrons that can be present in a neutral atom. The number of neutrons in an atom can vary, leading to different isotopes of an element.
📝 Note: This answer key is meant to serve as a guide for students and instructors. It is essential to review the worksheet questions and answers carefully to ensure accuracy and completeness.
Table: Subatomic Particles and Their Properties
| Subatomic Particle | Charge | Mass | Location |
|---|---|---|---|
| Proton | Positive | 1 atomic mass unit (amu) | Nucleus |
| Neutron | No charge | 1 amu | Nucleus |
| Electron | Negative | 1/1836 amu | Electron cloud |
In conclusion, understanding the atomic structure is crucial for grasping the fundamental principles of chemistry. By learning about the components of an atom, electron configuration, and more, students can develop a strong foundation in chemistry and prepare for future studies.
What is the difference between an atom and a molecule?
+An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of atoms that are chemically bonded together.
What is the atomic number of an element?
+The atomic number of an element is the number of protons present in the nucleus of an atom.
What is electron configuration?
+Electron configuration is a way of describing the arrangement of electrons in an atom.