5 Main Types of Chemical Reactions

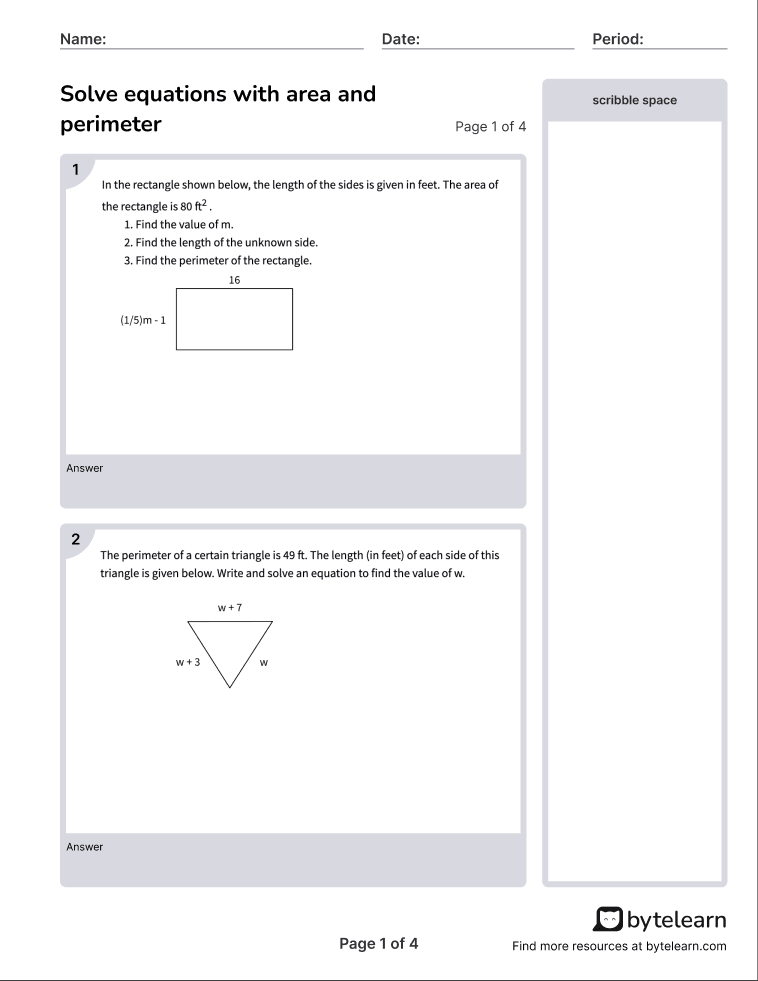
Understanding the Fundamentals of Chemical Reactions
Chemical reactions are the backbone of chemistry, and they occur all around us, from the simplest biological processes to the most complex industrial operations. A chemical reaction is a process where one or more substances (reactants) are converted into new substances (products). These reactions are classified into several types based on their characteristics, and understanding these types is crucial for grasping the fundamentals of chemistry.
The Five Main Types of Chemical Reactions
Chemical reactions are broadly classified into five main types: synthesis, decomposition, single displacement, double displacement, and combustion reactions. Each type has its unique characteristics and plays a vital role in various chemical processes.
1. Synthesis Reactions
Synthesis reactions, also known as combination reactions, are those in which two or more substances combine to form a new compound. These reactions are represented by the general equation:
A + B → AB
In this type of reaction, the reactants A and B combine to form a new product AB.
Example:
2H2 + O2 → 2H2O
In this example, hydrogen gas (H2) and oxygen gas (O2) combine to form water (H2O).
2. Decomposition Reactions
Decomposition reactions are those in which a single compound breaks down into two or more simpler substances. These reactions are represented by the general equation:
AB → A + B
In this type of reaction, the reactant AB breaks down into products A and B.
Example:
2H2O → 2H2 + O2
In this example, water (H2O) breaks down into hydrogen gas (H2) and oxygen gas (O2).
3. Single Displacement Reactions
Single displacement reactions, also known as substitution reactions, are those in which one element displaces another element from a compound. These reactions are represented by the general equation:
A + BC → AC + B
In this type of reaction, the reactant A displaces the element B from the compound BC, resulting in the formation of a new compound AC.
Example:
Zn + CuSO4 → ZnSO4 + Cu
In this example, zinc (Zn) displaces copper (Cu) from copper sulfate (CuSO4), resulting in the formation of zinc sulfate (ZnSO4) and copper (Cu).
4. Double Displacement Reactions
Double displacement reactions, also known as exchange reactions, are those in which two compounds exchange partners, resulting in the formation of two new compounds. These reactions are represented by the general equation:
AB + CD → AD + BC
In this type of reaction, the reactants AB and CD exchange partners, resulting in the formation of new compounds AD and BC.
Example:
NaCl + AgNO3 → NaNO3 + AgCl
In this example, sodium chloride (NaCl) and silver nitrate (AgNO3) exchange partners, resulting in the formation of sodium nitrate (NaNO3) and silver chloride (AgCl).
5. Combustion Reactions
Combustion reactions are those in which a substance reacts with oxygen, resulting in the release of heat and light. These reactions are represented by the general equation:
A + O2 → CO2 + H2O + heat + light
In this type of reaction, the reactant A reacts with oxygen (O2), resulting in the release of heat and light, and the formation of carbon dioxide (CO2) and water (H2O).
Example:
CH4 + 2O2 → CO2 + 2H2O + heat + light
In this example, methane (CH4) reacts with oxygen (O2), resulting in the release of heat and light, and the formation of carbon dioxide (CO2) and water (H2O).
🔥 Note: Combustion reactions are a special type of chemical reaction that involves the reaction of a substance with oxygen, resulting in the release of heat and light.
Conclusion
In conclusion, understanding the five main types of chemical reactions is essential for grasping the fundamentals of chemistry. Each type of reaction has its unique characteristics and plays a vital role in various chemical processes. By recognizing the different types of chemical reactions, we can better understand the world around us and develop new technologies and innovations.
What is a chemical reaction?
+A chemical reaction is a process where one or more substances (reactants) are converted into new substances (products).
What are the five main types of chemical reactions?
+The five main types of chemical reactions are synthesis, decomposition, single displacement, double displacement, and combustion reactions.
What is the general equation for a synthesis reaction?
+The general equation for a synthesis reaction is A + B → AB.
Related Terms:
- Chemical reactions Worksheet answers pdf
- Identifying chemical reactions Worksheet