Calculating Percent Abundance of Isotopes Made Easy

Understanding Percent Abundance of Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons in their atomic nuclei. The concept of percent abundance is crucial in understanding the natural occurrence of these isotopes. Percent abundance refers to the relative abundance of a particular isotope of an element in a naturally occurring sample. In this blog post, we will explore the concept of percent abundance, its importance, and how to calculate it.
Why is Percent Abundance Important?
Percent abundance is essential in various fields, including chemistry, physics, and geology. It helps scientists:
- Understand the natural occurrence of elements: By knowing the percent abundance of isotopes, scientists can understand the natural distribution of elements in the Earth’s crust, atmosphere, and oceans.
- Identify the origin of materials: Percent abundance can be used to determine the origin of materials, such as rocks, minerals, and fossils.
- Determine the age of rocks and minerals: By analyzing the percent abundance of radioactive isotopes, scientists can determine the age of rocks and minerals.
Calculating Percent Abundance
Calculating percent abundance is a straightforward process that involves the following steps:
- Determine the number of atoms of each isotope: Use a mass spectrometer or other analytical techniques to determine the number of atoms of each isotope in a sample.
- Calculate the total number of atoms: Add up the number of atoms of each isotope to get the total number of atoms in the sample.
- Calculate the percent abundance: Divide the number of atoms of each isotope by the total number of atoms and multiply by 100.
The formula for calculating percent abundance is:
Percent Abundance = (Number of atoms of isotope / Total number of atoms) x 100
Example: Calculating Percent Abundance of Carbon-12 and Carbon-13
Suppose we have a sample of carbon that contains 98.9% carbon-12 and 1.1% carbon-13. We can calculate the percent abundance of each isotope as follows:
- Number of atoms of carbon-12 = 98.9
- Number of atoms of carbon-13 = 1.1
- Total number of atoms = 98.9 + 1.1 = 100
Percent Abundance of carbon-12 = (98.9 / 100) x 100 = 98.9% Percent Abundance of carbon-13 = (1.1 / 100) x 100 = 1.1%
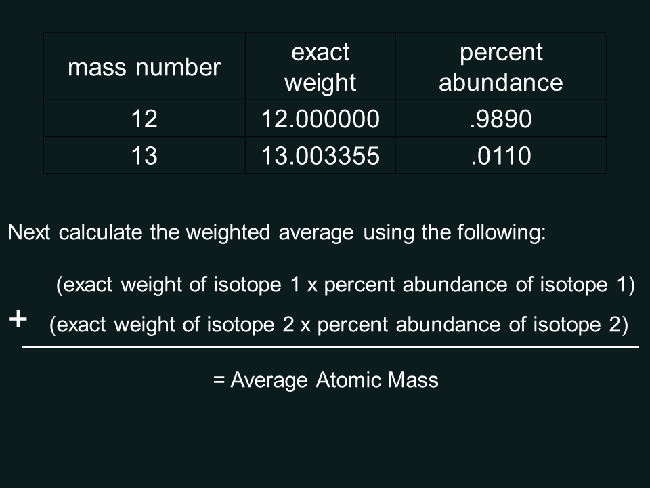
| Isotope | Number of Atoms | Percent Abundance |
|---|---|---|
| Carbon-12 | 98.9 | 98.9% |
| Carbon-13 | 1.1 | 1.1% |
📝 Note: The percent abundance values are usually expressed as a percentage, but they can also be expressed as a decimal or a fraction.
Common Applications of Percent Abundance
Percent abundance has numerous applications in various fields, including:
- Geology: Percent abundance is used to determine the age of rocks and minerals, identify the origin of materials, and understand the geological history of an area.
- Chemistry: Percent abundance is used to identify the isotopic composition of elements, understand the chemical properties of elements, and determine the authenticity of materials.
- Environmental Science: Percent abundance is used to study the movement of elements in the environment, understand the biogeochemical cycles of elements, and identify the sources of pollution.
Challenges and Limitations of Calculating Percent Abundance
Calculating percent abundance can be challenging due to the following limitations:
- Instrumental errors: The accuracy of the percent abundance values depends on the precision of the analytical instruments used.
- Sample size: The sample size can affect the accuracy of the percent abundance values.
- Interference from other elements: The presence of other elements can interfere with the analysis and affect the accuracy of the percent abundance values.
In summary, calculating percent abundance is a crucial step in understanding the natural occurrence of isotopes. By following the steps outlined in this blog post, scientists can accurately calculate the percent abundance of isotopes and gain valuable insights into the natural world.
What is the significance of percent abundance in geology?
+Percent abundance is significant in geology because it helps scientists determine the age of rocks and minerals, identify the origin of materials, and understand the geological history of an area.
How is percent abundance calculated?
+Percent abundance is calculated by dividing the number of atoms of each isotope by the total number of atoms and multiplying by 100.
What are the limitations of calculating percent abundance?
+The limitations of calculating percent abundance include instrumental errors, sample size, and interference from other elements.
Related Terms:
- Percentage abundance formula
- Isotope Practice Worksheet answers PDF