5 Ways to Master Boyle's Law with Worksheets
Understanding Boyle's Law
Boyle’s Law is a fundamental concept in chemistry and physics that describes the relationship between the pressure and volume of a gas. The law states that, at constant temperature, the volume of a gas is inversely proportional to the pressure. In other words, as the pressure of a gas increases, its volume decreases, and vice versa. This concept is crucial in understanding various natural phenomena and has numerous applications in fields like engineering, physics, and chemistry.
Breaking Down Boyle's Law
To master Boyle’s Law, it’s essential to understand the mathematical relationship between pressure and volume. The law can be expressed as:
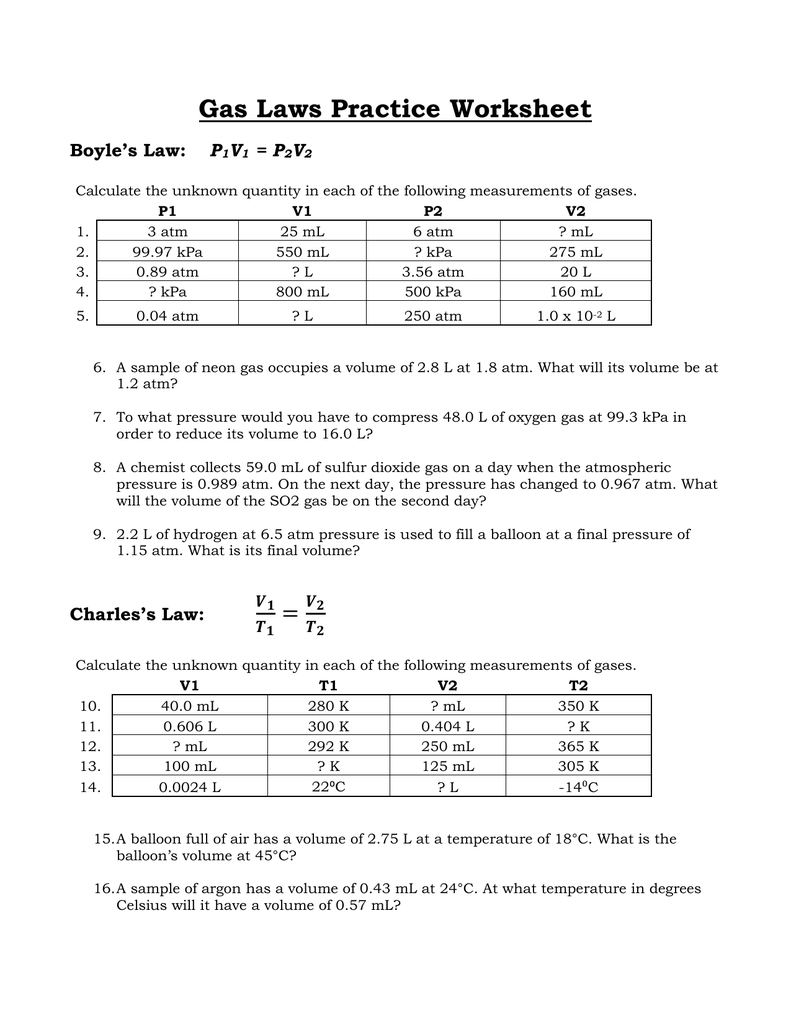
P1V1 = P2V2
Where:
- P1 is the initial pressure
- V1 is the initial volume
- P2 is the final pressure
- V2 is the final volume
This equation shows that the product of the initial pressure and volume is equal to the product of the final pressure and volume.
5 Ways to Master Boyle's Law with Worksheets
Mastering Boyle’s Law requires practice and application. Here are five ways to help you master the concept using worksheets:
1. Simple Calculations
Start by practicing simple calculations using the Boyle’s Law equation. You can use worksheets with given values for initial and final pressures and volumes. Calculate the unknown value using the equation.
For example:

| Initial Pressure (P1) | Initial Volume (V1) | Final Pressure (P2) | Final Volume (V2) |
|---|---|---|---|
| 2 atm | 10 L | 4 atm | ? |
Using the equation, calculate the final volume (V2).
Answer: V2 = (P1V1) / P2 = (2 atm x 10 L) / 4 atm = 5 L
2. Graphical Analysis
Graphical analysis is an excellent way to visualize the relationship between pressure and volume. Create a worksheet with a graph of pressure versus volume. Plot different points on the graph, and use the equation to calculate the corresponding values.
For example:
Plot the following points on a graph of pressure versus volume:
| Pressure (atm) | Volume (L) |
|---|---|
| 1 | 20 |
| 2 | 10 |
| 3 | 6.67 |
| 4 | 5 |
Use the graph to determine the relationship between pressure and volume.
3. Real-World Applications
Boyle’s Law has numerous real-world applications. Create worksheets that involve solving problems related to real-world scenarios, such as:
- A scuba diver descending to a depth of 20 meters, where the pressure is 3 times the atmospheric pressure. If the initial volume of the air in the tank is 10 L, what is the final volume?
- A pneumatic cylinder with an initial pressure of 5 atm and a volume of 20 L. If the pressure increases to 10 atm, what is the final volume?
4. Word Problems
Word problems are an excellent way to test your understanding of Boyle’s Law. Create worksheets with word problems that involve solving for unknown values using the equation.
For example:
“A gas cylinder has an initial pressure of 2 atm and a volume of 15 L. If the pressure increases to 4 atm, what is the final volume?”
5. Mixed-Concept Problems
Mixed-concept problems involve solving for multiple unknown values using Boyle’s Law and other concepts, such as temperature and number of moles. Create worksheets with mixed-concept problems to challenge yourself.
For example:
“A gas sample has an initial pressure of 3 atm, a volume of 20 L, and a temperature of 25°C. If the temperature increases to 35°C and the pressure increases to 6 atm, what is the final volume?”
Important Notes
When working with Boyle’s Law, keep the following notes in mind:
📝 Note: Boyle's Law assumes a constant temperature. If the temperature changes, the law does not apply.
📝 Note: Boyle's Law is only applicable to ideal gases. Real gases may deviate from the law due to intermolecular forces.
By following these five ways to master Boyle’s Law with worksheets, you’ll become proficient in applying the concept to various problems and scenarios.
Boyle’s Law is a fundamental concept in chemistry and physics, and mastering it requires practice and application. By using worksheets and practicing different types of problems, you’ll become proficient in applying the concept to various scenarios. Remember to keep the notes in mind when working with Boyle’s Law, and you’ll be well on your way to mastering the concept.
What is Boyle’s Law?
+
Boyle’s Law states that, at constant temperature, the volume of a gas is inversely proportional to the pressure.
What is the mathematical equation for Boyle’s Law?
+
The equation for Boyle’s Law is P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
What are some real-world applications of Boyle’s Law?
+
Boyle’s Law has numerous real-world applications, including scuba diving, pneumatic systems, and industrial processes.



