Bohr Model Worksheet With Answers: Key to Atomic Understanding
Unlocking the Secrets of the Atom: A Comprehensive Guide to the Bohr Model
The Bohr model, proposed by Niels Bohr in 1913, is a fundamental concept in understanding the structure of atoms. This model revolutionized the field of physics and chemistry, providing a simple yet effective way to visualize the atom. In this article, we will delve into the world of the Bohr model, exploring its key components, advantages, and limitations.
What is the Bohr Model?
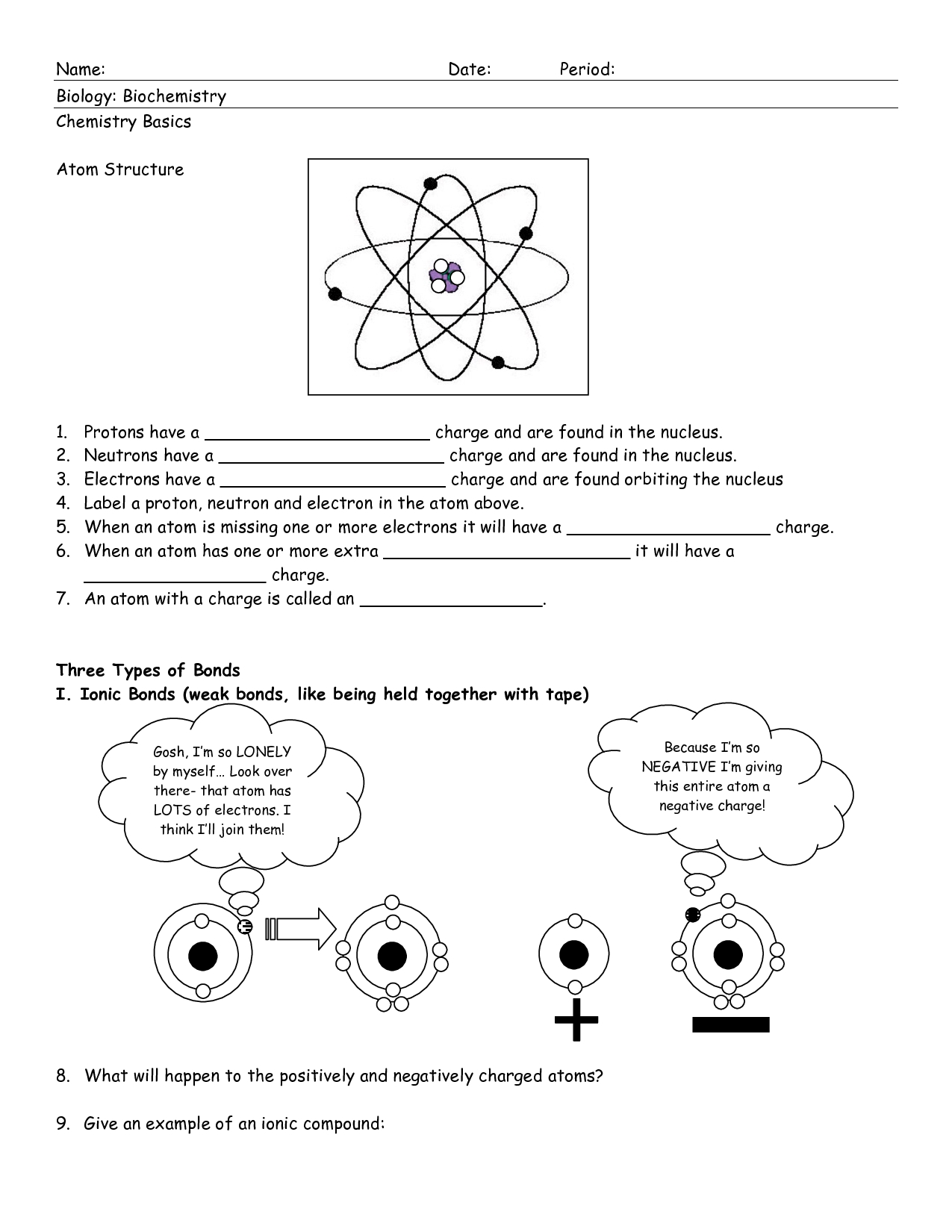
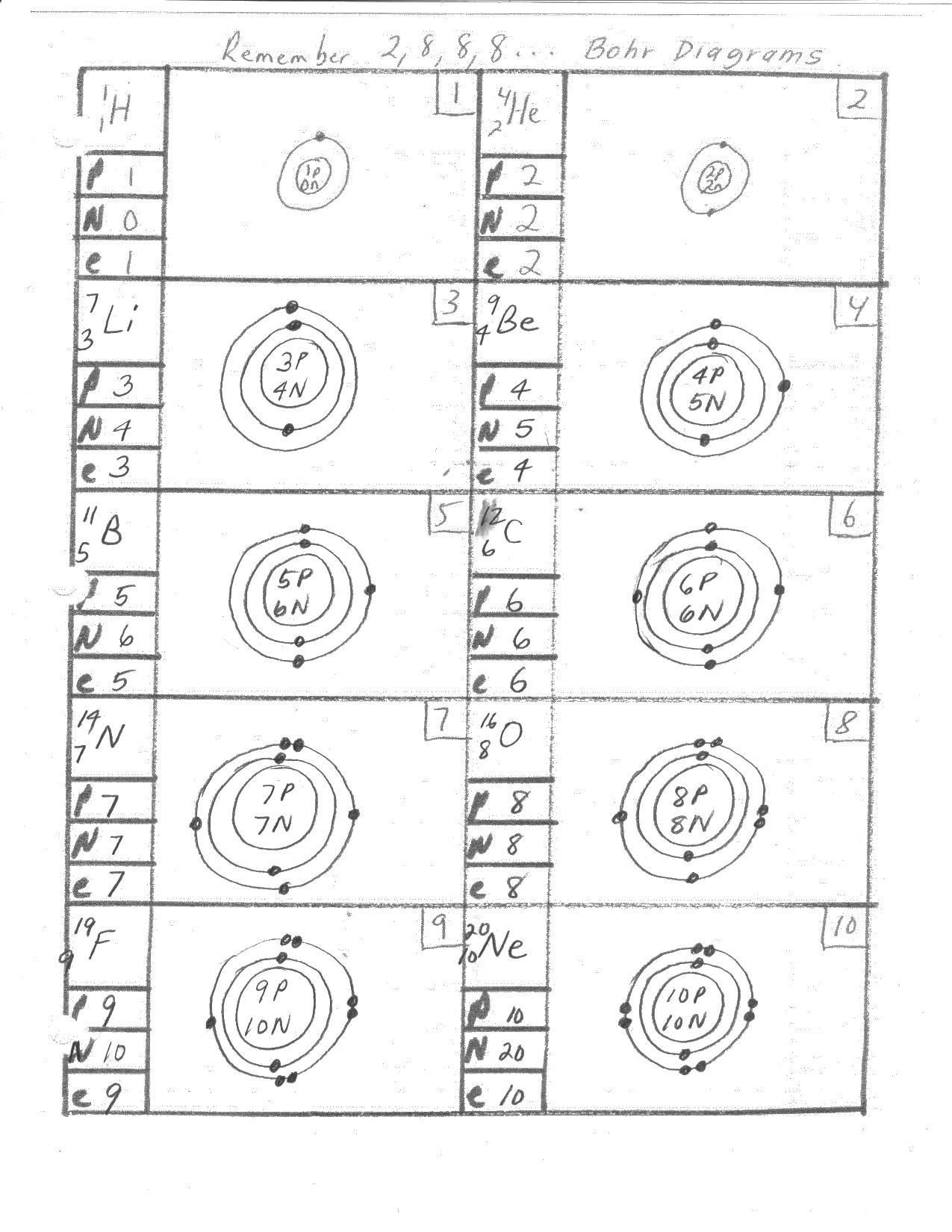
The Bohr model is a simplified representation of the atom, consisting of a small, heavy nucleus surrounded by electrons in circular orbits. The model is based on the following key assumptions:
- Electrons occupy specific energy levels: Electrons are arranged in fixed, circular orbits around the nucleus, each corresponding to a specific energy level.
- Energy levels are quantized: Electrons can only occupy specific energy levels, and each level has a specific energy value.
- Electrons jump between energy levels: Electrons can jump from one energy level to another by emitting or absorbing energy in the form of photons.
Key Components of the Bohr Model
The Bohr model consists of several key components, including:
- Nucleus: The central, positively charged region of the atom, composed of protons and neutrons.
- Electrons: Negatively charged particles that orbit the nucleus in circular paths.
- Energy levels: Specific regions around the nucleus where electrons are found, each corresponding to a specific energy value.
- Electron shells: Regions around the nucleus where electrons are found, divided into different energy levels.
Advantages of the Bohr Model
The Bohr model has several advantages, including:
- Simplicity: The model provides a simple, intuitive way to visualize the atom.
- Predictive power: The model can predict the energy levels and electron configuration of atoms.
- Foundation for quantum mechanics: The Bohr model laid the foundation for the development of quantum mechanics.
Limitations of the Bohr Model
While the Bohr model is a powerful tool for understanding the atom, it has several limitations, including:
- Oversimplification: The model oversimplifies the complexity of atomic structure.
- Inability to explain atomic phenomena: The model cannot explain certain atomic phenomena, such as the Zeeman effect.
Bohr Model Worksheet With Answers
Section 1: Multiple Choice Questions
- Who proposed the Bohr model in 1913? a) Niels Bohr b) Ernest Rutherford c) Albert Einstein d) Max Planck
Answer: a) Niels Bohr
- What is the main assumption of the Bohr model? a) Electrons occupy specific energy levels b) Energy levels are continuous c) Electrons are found in a cloud around the nucleus d) The nucleus is negatively charged
Answer: a) Electrons occupy specific energy levels
Section 2: Short Answer Questions
- Describe the structure of the atom according to the Bohr model.
Answer: The atom consists of a small, heavy nucleus surrounded by electrons in circular orbits. Each orbit corresponds to a specific energy level.
- What is the difference between an energy level and an electron shell?
Answer: An energy level is a specific region around the nucleus where electrons are found, while an electron shell is a region around the nucleus divided into different energy levels.
Section 3: Essay Questions
- Describe the advantages and limitations of the Bohr model.
Answer: The Bohr model provides a simple, intuitive way to visualize the atom and can predict the energy levels and electron configuration of atoms. However, the model oversimplifies the complexity of atomic structure and cannot explain certain atomic phenomena.
- How did the Bohr model lay the foundation for the development of quantum mechanics?
Answer: The Bohr model introduced the concept of quantized energy levels and electron jumps between energy levels, which laid the foundation for the development of quantum mechanics.
💡 Note: The Bohr model is a simplified representation of the atom and should not be taken as a literal representation of atomic structure.
In conclusion, the Bohr model is a fundamental concept in understanding the structure of atoms. While it has its limitations, the model provides a simple, intuitive way to visualize the atom and has laid the foundation for the development of quantum mechanics. By understanding the Bohr model, we can gain a deeper appreciation for the complexity and beauty of the atomic world.
What is the main assumption of the Bohr model?
+The main assumption of the Bohr model is that electrons occupy specific energy levels.
What is the difference between an energy level and an electron shell?
+An energy level is a specific region around the nucleus where electrons are found, while an electron shell is a region around the nucleus divided into different energy levels.
How did the Bohr model lay the foundation for the development of quantum mechanics?
+The Bohr model introduced the concept of quantized energy levels and electron jumps between energy levels, which laid the foundation for the development of quantum mechanics.