Bohr Model Diagrams Worksheet Answers Explained
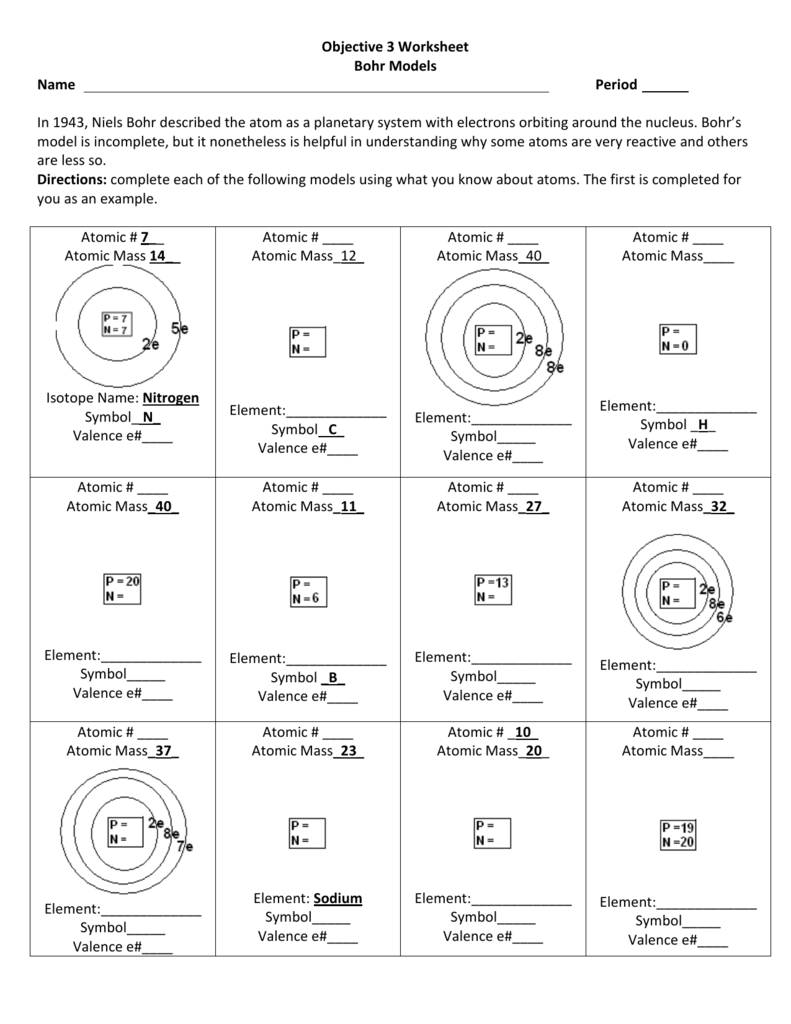
Understanding the Bohr Model Diagrams Worksheet Answers
The Bohr model is a fundamental concept in chemistry that describes the structure of an atom. It was developed by Niels Bohr in 1913 and is still widely used today to understand the behavior of electrons in an atom. In this post, we will explain the answers to a Bohr model diagrams worksheet and provide a detailed understanding of the concept.
What is the Bohr Model?
The Bohr model is a simplified representation of an atom that describes the arrangement of electrons around the nucleus. According to the Bohr model, electrons occupy specific energy levels or shells around the nucleus. Each energy level has a specific capacity, and electrons in each energy level have a specific amount of energy.
Bohr Model Diagrams Worksheet Answers Explained
Here are the answers to a Bohr model diagrams worksheet, along with explanations:
Question 1: What is the maximum number of electrons that can occupy the first energy level?
Answer: 2
Explanation: The first energy level, also known as the s-orbital, can accommodate a maximum of 2 electrons.
Question 2: What is the maximum number of electrons that can occupy the second energy level?
Answer: 8
Explanation: The second energy level, also known as the s- and p-orbitals, can accommodate a maximum of 8 electrons.
Question 3: Draw the Bohr model diagram for a hydrogen atom.
Answer:
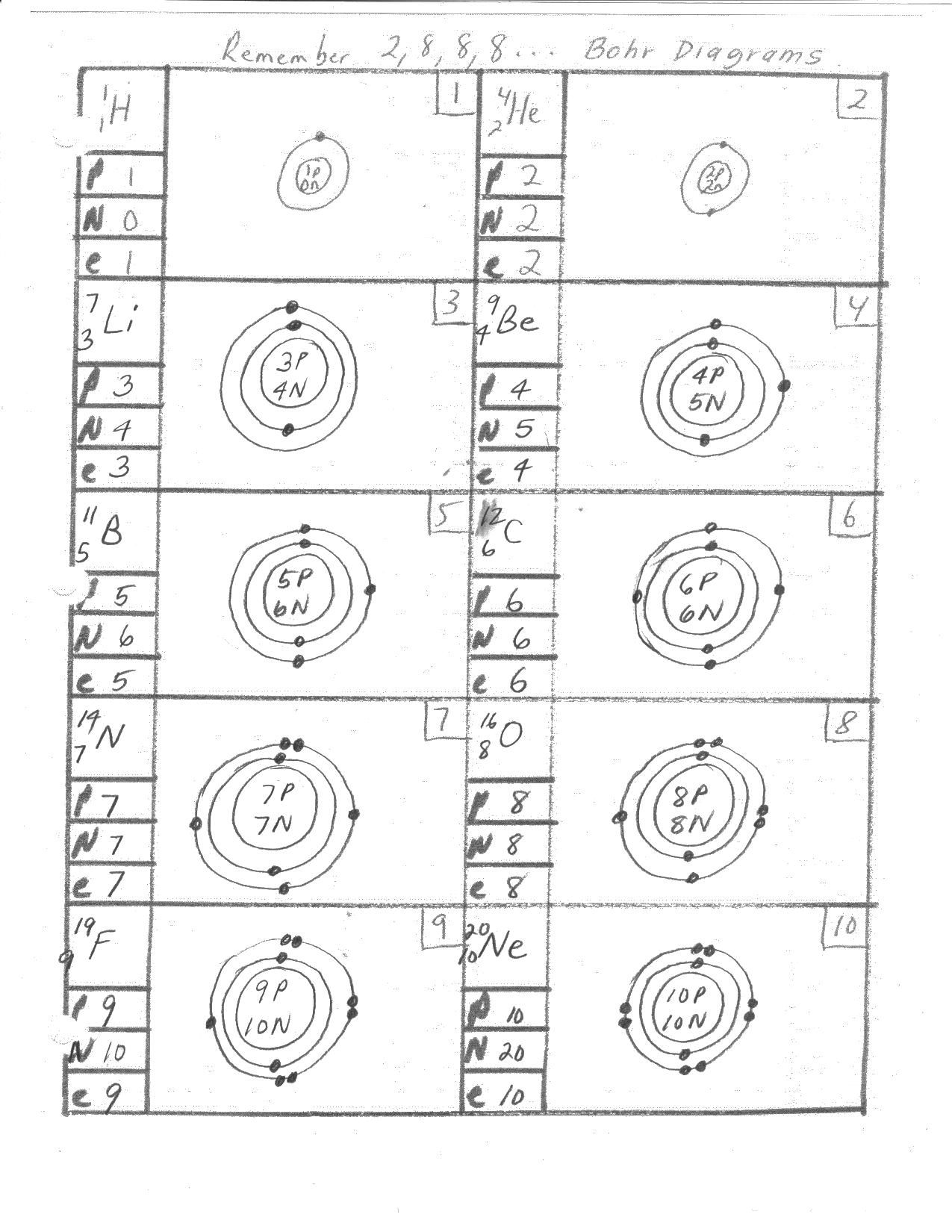
| Energy Level | Electrons |
| 1 | 1 |
| 2 | 0 |
| 3 | 0 |
Explanation: A hydrogen atom has only one proton in its nucleus and one electron in the first energy level.
Question 4: Draw the Bohr model diagram for a helium atom.
Answer:
| Energy Level | Electrons |
| 1 | 2 |
| 2 | 0 |
| 3 | 0 |
Explanation: A helium atom has two protons in its nucleus and two electrons in the first energy level.
Important Notes
- The Bohr model is a simplified representation of an atom and does not account for the complexities of atomic structure.
- The Bohr model is still widely used today to understand the behavior of electrons in an atom.
- The Bohr model is not suitable for explaining the behavior of electrons in multi-electron atoms.
💡 Note: The Bohr model is a fundamental concept in chemistry, and understanding it is essential for understanding the behavior of atoms and molecules.
Common Mistakes to Avoid
- Drawing the Bohr model diagram with incorrect energy levels or electron configurations.
- Not understanding the maximum capacity of each energy level.
- Not drawing the correct number of electrons in each energy level.
🚫 Note: Avoid drawing the Bohr model diagram with incorrect energy levels or electron configurations, as this can lead to incorrect conclusions about the behavior of electrons in an atom.
Conclusion
In conclusion, the Bohr model is a fundamental concept in chemistry that describes the structure of an atom. Understanding the Bohr model diagrams worksheet answers requires a basic understanding of the concept and the ability to draw correct diagrams. By following the explanations provided in this post, you can improve your understanding of the Bohr model and avoid common mistakes.
What is the Bohr model?
+The Bohr model is a simplified representation of an atom that describes the arrangement of electrons around the nucleus.
What is the maximum number of electrons that can occupy the first energy level?
+The maximum number of electrons that can occupy the first energy level is 2.
How do I draw a Bohr model diagram?
+To draw a Bohr model diagram, start by drawing a small circle to represent the nucleus, and then draw energy levels around the nucleus. Each energy level should have a specific capacity, and electrons should be drawn in each energy level.
Related Terms:
- Bohr Model diagram worksheet pdf
- Bohr model valence electrons Worksheet
- Bohr model Practice
- Bohr model Worksheet Doc