5 Steps to Master Balancing Equations
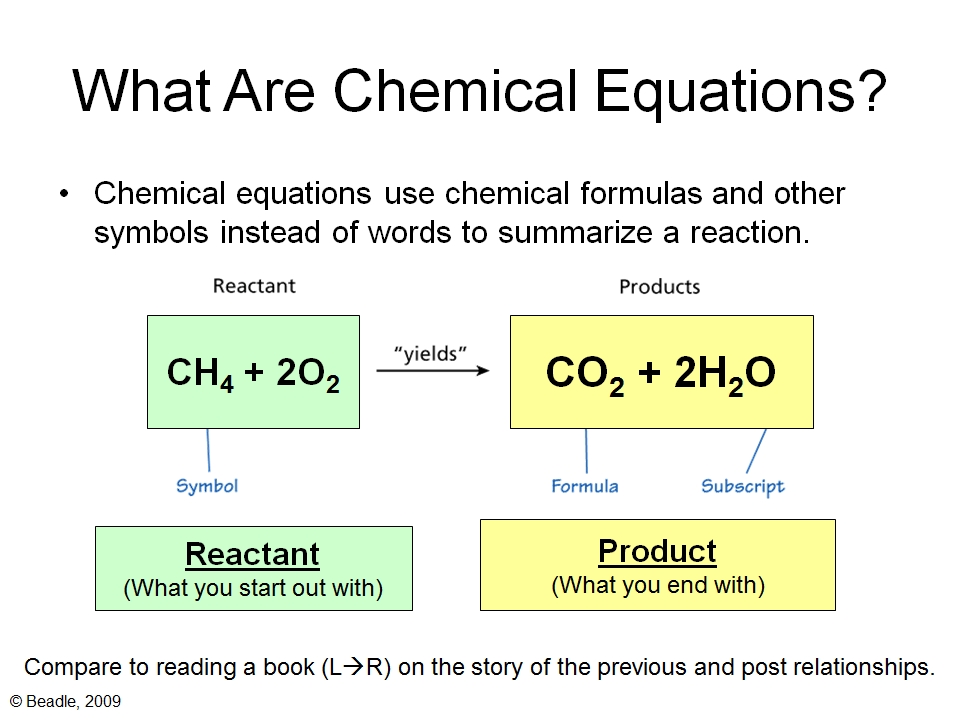
Step 1: Understand the Basics of Chemical Equations
Balancing chemical equations is a fundamental concept in chemistry that represents the law of conservation of mass. It states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, you need to ensure that the number of atoms of each element is the same on both the reactant and product sides. Chemical equations are written in a specific format, with reactants on the left and products on the right, separated by an arrow.
To start balancing an equation, write down the unbalanced equation with the reactants on the left and the products on the right. For example:
Na + O2 → Na2O
This equation represents the reaction between sodium (Na) and oxygen (O2) to form sodium oxide (Na2O).
🔥 Note: Make sure to write the equation with the correct chemical formulas and reactants.
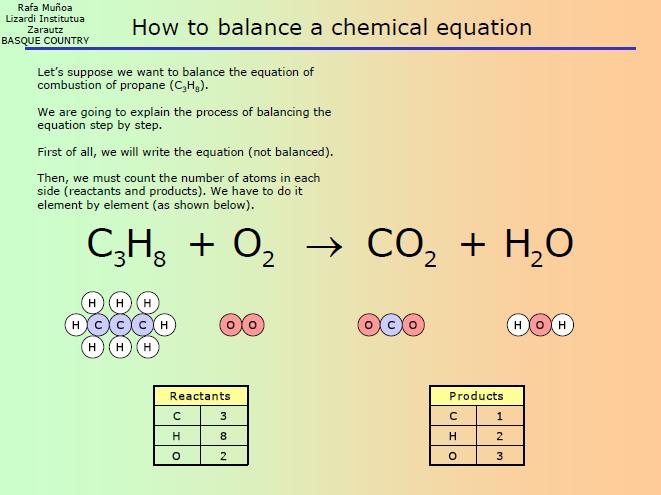
Step 2: Count the Atoms of Each Element
Count the number of atoms of each element on both the reactant and product sides. This step is crucial in identifying which elements need to be balanced.
| Element | Reactant Side | Product Side |
|---|---|---|
| Na | 1 | 2 |
| O | 2 | 1 |
From the table above, we can see that sodium (Na) has 1 atom on the reactant side and 2 atoms on the product side. Oxygen (O) has 2 atoms on the reactant side and 1 atom on the product side.
Step 3: Balance the Atoms of Each Element
To balance the atoms of each element, we need to add coefficients (numbers in front of the formulas of reactants or products) to ensure that the number of atoms of each element is the same on both sides.
- To balance sodium (Na), we add a coefficient of 2 in front of Na on the reactant side: 2Na + O2 → Na2O
- To balance oxygen (O), we add a coefficient of 2 in front of O2 on the reactant side is not necessary, instead, we add a coefficient of 1⁄2 in front of O2, but since we cannot have a fraction as a coefficient, we multiply the entire equation by 2: 4Na + 2O2 → 2Na2O
Step 4: Check the Balanced Equation
Once you have added coefficients to balance the atoms of each element, re-count the atoms to ensure that the equation is balanced.
| Element | Reactant Side | Product Side |
|---|---|---|
| Na | 4 | 4 |
| O | 4 | 4 |
From the table above, we can see that the number of atoms of each element is the same on both sides, indicating that the equation is balanced.
🔍 Note: Always check the balanced equation to ensure that it is correct.
Step 5: Practice, Practice, Practice!
Balancing chemical equations requires practice to become proficient. Start with simple equations and gradually move on to more complex ones.
Some tips to keep in mind:
- Start with simple equations: Begin with equations that have fewer reactants and products.
- Use a systematic approach: Follow the steps outlined above to ensure that you balance the equation correctly.
- Check your work: Re-count the atoms to ensure that the equation is balanced.
By following these steps and practicing regularly, you will become proficient in balancing chemical equations.
Balancing chemical equations is a fundamental concept in chemistry that requires practice to become proficient. By following the steps outlined above, you can master the art of balancing chemical equations.
Here are some key points to summarize:
- Understand the basics of chemical equations and the law of conservation of mass.
- Count the atoms of each element on both the reactant and product sides.
- Balance the atoms of each element by adding coefficients.
- Check the balanced equation to ensure that it is correct.
- Practice regularly to become proficient.
If you have any questions or need further clarification, please refer to the FAQ section below.
What is the law of conservation of mass?
+The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
What is a coefficient in a chemical equation?
+A coefficient is a number placed in front of the formula of a reactant or product in a chemical equation.
How do I know if a chemical equation is balanced?
+A chemical equation is balanced when the number of atoms of each element is the same on both the reactant and product sides.