5 Easy Steps to Balance Chemical Equations
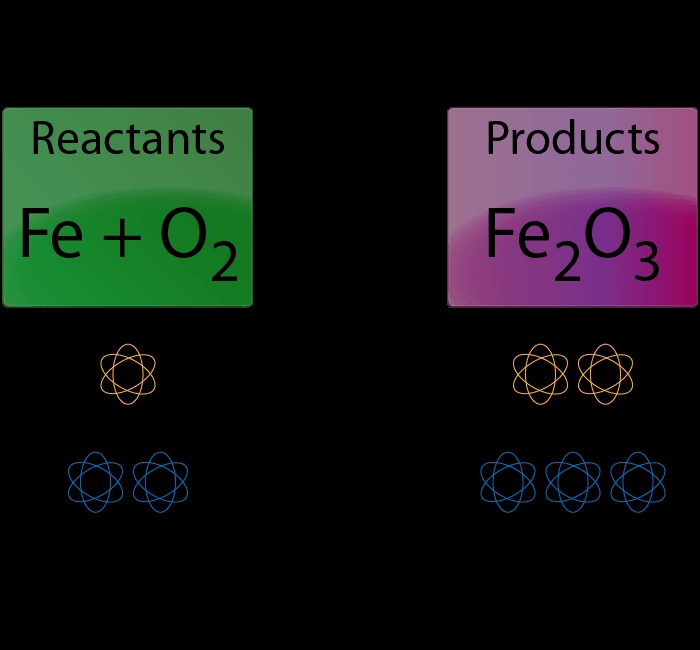
Chemical equations are a crucial part of chemistry, representing the reactions that occur between different substances. However, these equations need to be balanced to ensure they accurately reflect the laws of chemistry, particularly the law of conservation of mass. In this post, we will explore a step-by-step guide on how to balance chemical equations easily.
Step 1: Write Down the Unbalanced Equation
The first step in balancing a chemical equation is to write down the unbalanced equation. This involves writing the reactants on the left side of the equation and the products on the right side. Make sure to include the chemical formulas of all reactants and products.
Example:
Unbalanced equation: Na + Cl2 → NaCl
Step 2: Count the Atoms of Each Element
Next, count the atoms of each element on both the reactant and product sides of the equation. This will help you identify which elements are unbalanced.
Example:
Reactants: Na (1), Cl2 (2) Products: NaCl (1 Na, 1 Cl)
From the example above, we can see that sodium (Na) is balanced, but chlorine (Cl) is not.
Step 3: Balance the Unbalanced Elements
To balance the unbalanced elements, we need to add coefficients in front of the formulas of the reactants or products. Coefficients are numbers that multiply the entire formula, not just one atom. Start by balancing elements that appear only once on each side of the equation.
Example:
To balance chlorine (Cl), we add a coefficient of 2 in front of NaCl:
Unbalanced equation: Na + Cl2 → 2NaCl
Now, we need to balance sodium (Na). Since there are 2 sodium atoms on the product side, we add a coefficient of 2 in front of Na:
Balanced equation: 2Na + Cl2 → 2NaCl
Step 4: Check the Balance of Each Element
Once you have added coefficients to balance the elements, re-count the atoms of each element on both sides of the equation to ensure that the equation is now balanced.
Example:
Reactants: 2Na (2), Cl2 (2) Products: 2NaCl (2 Na, 2 Cl)
The equation is now balanced.
Step 5: Write the Final Balanced Equation
The final step is to write the final balanced equation. Make sure to include all the coefficients and chemical formulas.
Example:
Balanced equation: 2Na + Cl2 → 2NaCl
Tips for Balancing Chemical Equations:
- Balance elements that appear only once on each side of the equation first.
- Use coefficients to balance elements, not subscripts.
- Re-count the atoms of each element after adding coefficients to ensure the equation is balanced.
- Check the balance of each element, not just the number of atoms.
Common Mistakes to Avoid:
- Changing subscripts: Never change the subscripts of a formula to balance an equation. This would result in a different compound.
- Forgetting to balance all elements: Make sure to balance all elements, not just the ones that are obviously unbalanced.
- Using too many coefficients: Try to use the smallest coefficients possible to balance the equation.
By following these 5 easy steps, you can balance chemical equations with ease and confidence.
Also, keep in mind that balancing chemical equations is a skill that takes practice, so don’t be discouraged if it takes time to get the hang of it. With time and practice, you will become proficient in balancing chemical equations.
What is the law of conservation of mass?
+
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants must equal the total mass of the products.
Why is it important to balance chemical equations?
+
Balancing chemical equations is important because it ensures that the equation accurately reflects the chemical reaction. An unbalanced equation can lead to incorrect calculations and conclusions.
Can I change the subscripts of a formula to balance an equation?
+
No, changing the subscripts of a formula would result in a different compound. Instead, use coefficients to balance the elements.