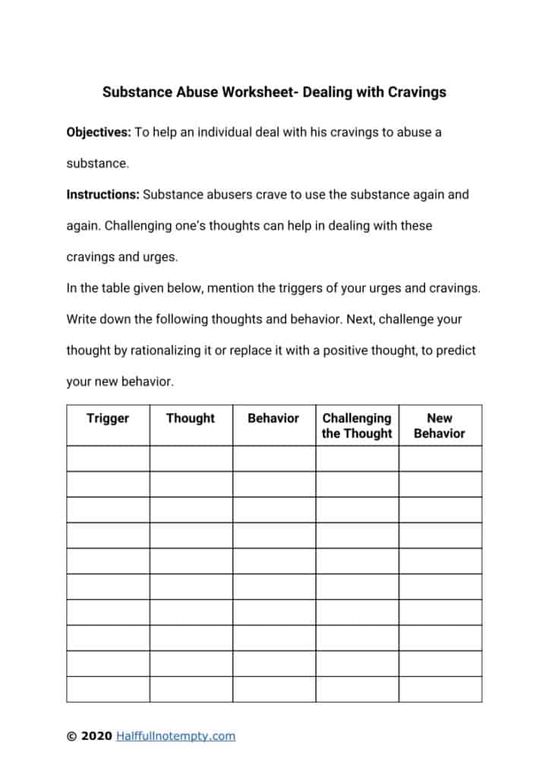
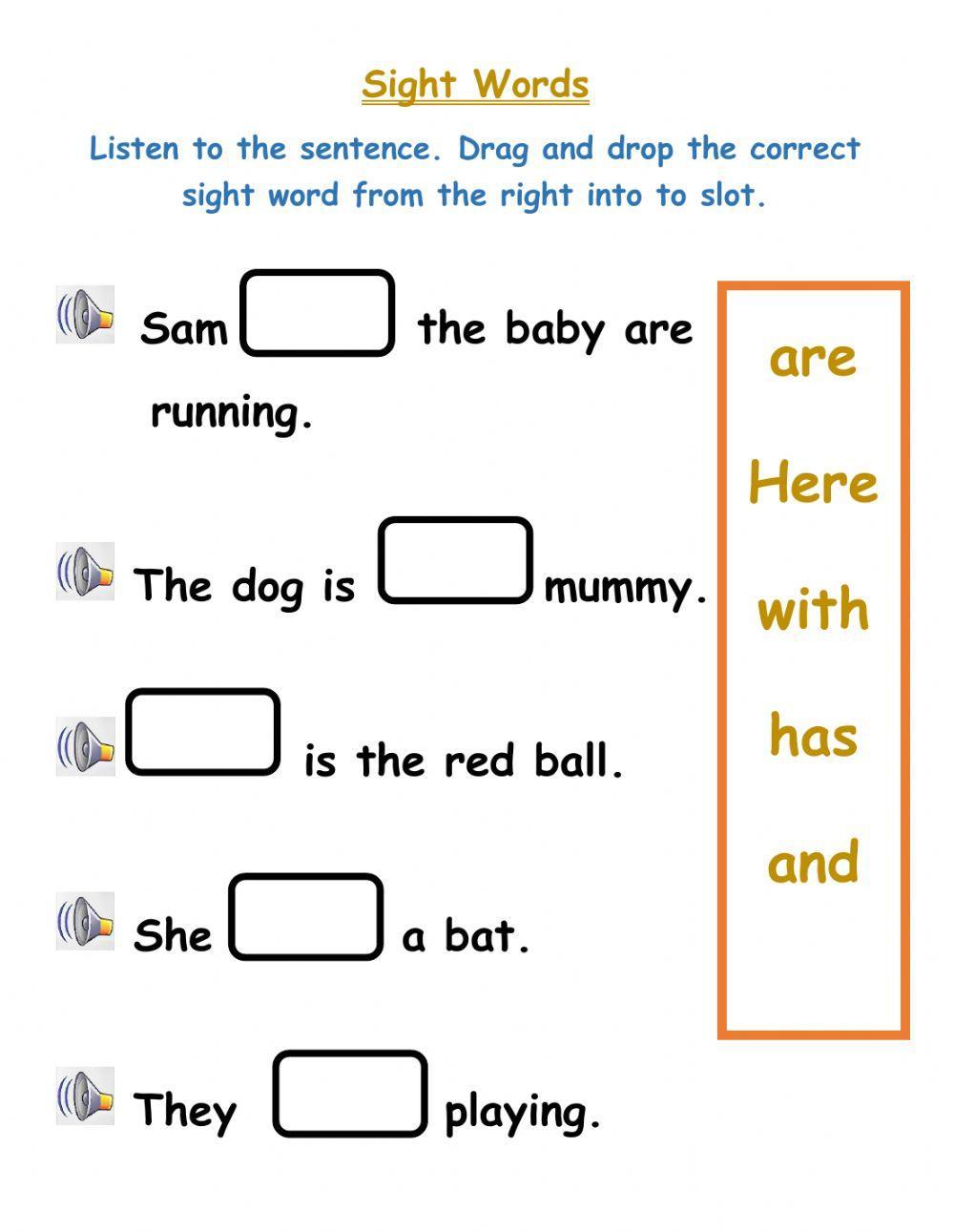
Balance Chemical Equations with Ease Worksheet 1

Introduction to Balancing Chemical Equations
Balancing chemical equations is a fundamental skill in chemistry that requires a deep understanding of the subject. It is a crucial step in writing chemical reactions, as it ensures that the law of conservation of mass is obeyed. In this article, we will provide a comprehensive guide on how to balance chemical equations with ease, along with a worksheet to practice your skills.
Why Balance Chemical Equations?
Balancing chemical equations is essential for several reasons:
- Law of Conservation of Mass: The law states that matter cannot be created or destroyed in a chemical reaction. Balancing chemical equations ensures that the number of atoms of each element is the same on both the reactant and product sides.
- Accurate Stoichiometry: Balanced chemical equations provide accurate stoichiometric ratios, which are essential for calculating the amount of reactants and products in a reaction.
- Predicting Products: Balanced chemical equations help predict the products of a reaction, which is crucial in understanding the outcome of a reaction.
Steps to Balance Chemical Equations
Balancing chemical equations involves a series of steps that can be followed to ensure that the equation is balanced. Here are the steps:
- Write the Unbalanced Equation: Start by writing the unbalanced equation, including all reactants and products.
- Count the Atoms: Count the number of atoms of each element on both the reactant and product sides.
- Identify the Imbalance: Identify the elements that are not balanced, i.e., the elements that have a different number of atoms on the reactant and product sides.
- Add Coefficients: Add coefficients (numbers in front of the formulas of reactants or products) to balance the elements that are not balanced.
- Check the Balance: Check the balance of the equation by counting the atoms of each element again.
- Repeat the Process: Repeat the process until the equation is balanced.
Worksheet 1: Balancing Chemical Equations
Here is a worksheet with five unbalanced chemical equations for you to practice balancing:

| Equation Number | Unbalanced Equation |
|---|---|
| 1 | Ca + HCl → CaCl2 + H2 |
| 2 | Al + Fe2O3 → Al2O3 + Fe |
| 3 | CH4 + O2 → CO2 + H2O |
| 4 | Na + H2O → NaOH + H2 |
| 5 | Zn + CuSO4 → ZnSO4 + Cu |
Balance each equation by adding coefficients and checking the balance.
Solution to Worksheet 1
Here are the solutions to the worksheet:
| Equation Number | Balanced Equation |
|---|---|
| 1 | Ca + 2HCl → CaCl2 + H2 |
| 2 | 2Al + Fe2O3 → Al2O3 + 2Fe |
| 3 | CH4 + 2O2 → CO2 + 2H2O |
| 4 | 2Na + 2H2O → 2NaOH + H2 |
| 5 | Zn + CuSO4 → ZnSO4 + Cu |
Conclusion
Balancing chemical equations is a crucial skill in chemistry that requires practice and patience. By following the steps outlined in this article and practicing with worksheets, you can become proficient in balancing chemical equations. Remember to always check the balance of the equation by counting the atoms of each element.
What is the purpose of balancing chemical equations?
+The purpose of balancing chemical equations is to ensure that the law of conservation of mass is obeyed, and to provide accurate stoichiometric ratios.
What are the steps to balance a chemical equation?
+The steps to balance a chemical equation are: write the unbalanced equation, count the atoms, identify the imbalance, add coefficients, check the balance, and repeat the process until the equation is balanced.
Why is it important to check the balance of a chemical equation?
+It is important to check the balance of a chemical equation to ensure that the law of conservation of mass is obeyed, and to provide accurate stoichiometric ratios.
Related Terms:
- Balancing chemical equations calculator
- Chemistry worksheets With Answers PDF
- Balancing chemical equations Worksheet PDF
- Writing skeleton equations Worksheet
- Word equations chemistry Worksheet answers