5 Steps to Master Balanced Equations
Mastering balanced equations is a crucial skill for any chemistry student. It’s a fundamental concept that can be challenging to grasp at first, but with practice and patience, you can become proficient in writing balanced equations. In this article, we’ll break down the process into five manageable steps, providing you with a clear understanding of how to balance equations like a pro.
Step 1: Understand the Basics
Before diving into the balancing process, it’s essential to understand the basics of chemical equations. A chemical equation represents a chemical reaction, where reactants are converted into products. The equation consists of:
- Reactants: The substances that undergo a chemical change.
- Products: The substances formed as a result of the chemical change.
- Arrow: Indicates the direction of the reaction.
- Coefficients: Numbers placed in front of the formulas of reactants or products to balance the equation.
- Subscripts: Small numbers written to the right of the chemical symbol, indicating the number of atoms of an element in a molecule.
Step 2: Write the Unbalanced Equation
The next step is to write the unbalanced equation, which involves:
- Identifying the reactants and products: Write the chemical formulas of the reactants on the left side of the arrow and the products on the right side.
- Using correct chemical formulas: Ensure that the chemical formulas are accurate, including the correct number of atoms and charges.
For example, let’s consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
H2 + O2 → H2O
Step 3: Count the Atoms
Now, it’s time to count the atoms of each element on both sides of the equation. This step is crucial in identifying the imbalance.
| Element | Reactants | Products |
|---|---|---|
| Hydrogen (H) | 2 | 2 |
| Oxygen (O) | 2 | 1 |
As you can see, there are 2 hydrogen atoms on both sides, but there’s an imbalance in oxygen atoms.
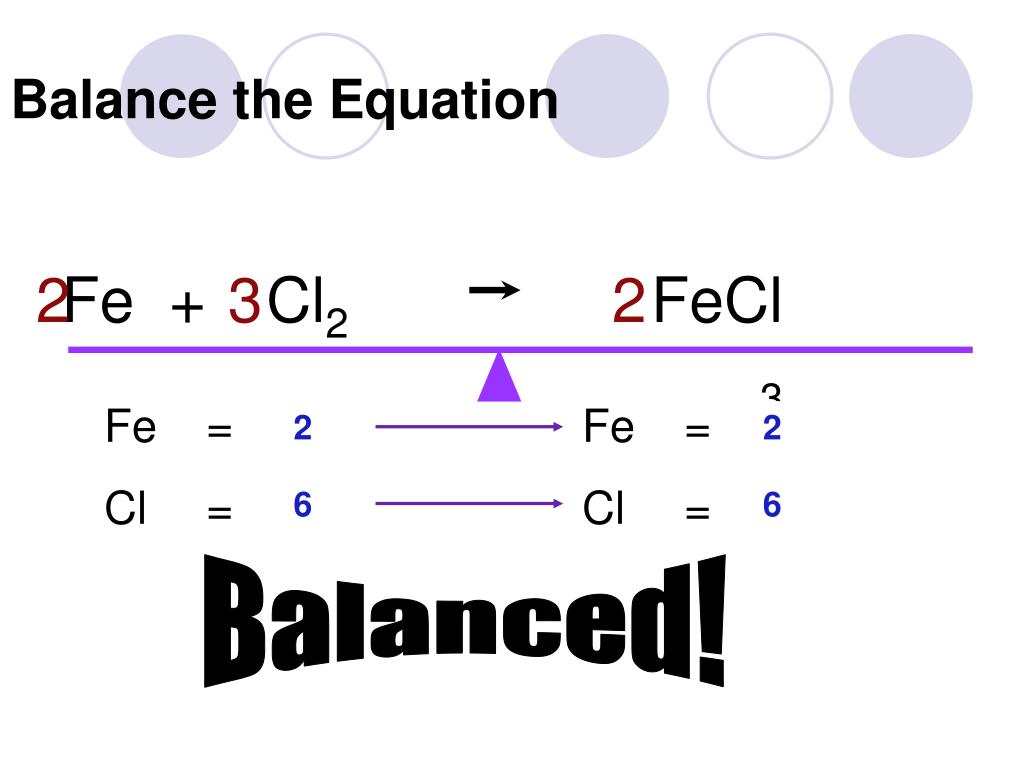
Step 4: Balance the Equation
To balance the equation, you need to add coefficients in front of the formulas of the reactants or products. The goal is to make the number of atoms of each element equal on both sides.
| Element | Reactants | Products |
|---|---|---|
| Hydrogen (H) | 2 | 2 |
| Oxygen (O) | 2 | 2 |
To balance the oxygen atoms, you can add a coefficient of 2 in front of the water molecule:
H2 + O2 → 2H2O
However, this would create an imbalance in hydrogen atoms. To fix this, you can add a coefficient of 2 in front of the hydrogen gas:
2H2 + O2 → 2H2O
Now, the equation is balanced.
Step 5: Verify the Balance
The final step is to verify that the equation is indeed balanced. Re-count the atoms of each element on both sides to ensure they match:
| Element | Reactants | Products |
|---|---|---|
| Hydrogen (H) | 4 | 4 |
| Oxygen (O) | 2 | 2 |
Congratulations! You’ve successfully balanced the equation.
📝 Note: When balancing equations, always check your work by re-counting the atoms. It's easy to make mistakes, so double-checking is crucial.
Common Mistakes to Avoid
When balancing equations, it’s essential to avoid common mistakes, such as:
- Changing subscripts: Never change the subscripts of a chemical formula to balance an equation.
- Adding or removing atoms: Avoid adding or removing atoms from the reactants or products to balance the equation.
- Forgetting to count atoms: Always count the atoms of each element on both sides of the equation.
By following these steps and avoiding common mistakes, you’ll become proficient in writing balanced equations. Remember to practice regularly to reinforce your understanding of this fundamental concept in chemistry.
As you master balanced equations, you’ll find that solving chemical problems becomes more manageable. You’ll be able to:
- Predict the products of a reaction: By understanding the reactants and the type of reaction, you can predict the products.
- Determine the limiting reactant: By balancing the equation, you can identify the limiting reactant, which is essential in calculating the yield of a reaction.
- Solve stoichiometry problems: Balancing equations is a critical step in solving stoichiometry problems, which involve calculating the amount of reactants or products in a reaction.
By mastering balanced equations, you’ll gain a deeper understanding of chemical reactions and be well-prepared to tackle more advanced topics in chemistry.
What is the importance of balancing chemical equations?
+
Balancing chemical equations is crucial in understanding the stoichiometry of a reaction, predicting the products, and determining the limiting reactant.
What are some common mistakes to avoid when balancing equations?
+
Avoid changing subscripts, adding or removing atoms, and forgetting to count atoms. These mistakes can lead to incorrect balancing and incorrect conclusions.
How can I practice balancing equations?
+
Practice balancing equations regularly using worksheets, online resources, or textbooks. Start with simple equations and gradually move on to more complex ones.