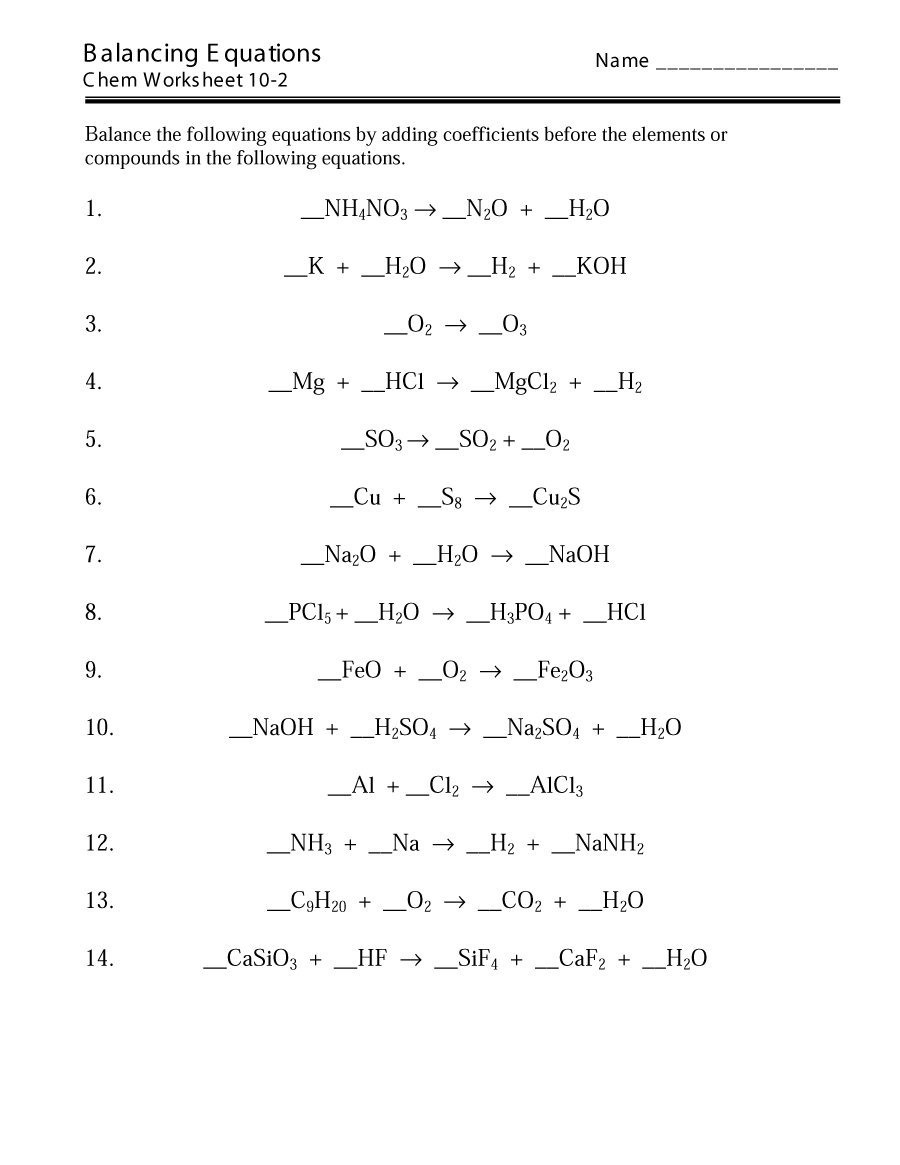
Balanced Chemical Equations Worksheet Answers and Solutions
Introduction to Balanced Chemical Equations
Balanced chemical equations are a fundamental concept in chemistry, representing the reaction between reactants and products in a way that conserves mass and follows the law of conservation of mass. In this worksheet, we will provide answers and solutions to help you better understand balanced chemical equations.
What are Balanced Chemical Equations?
Balanced chemical equations are equations that show the reactants and products of a chemical reaction, with the number of atoms of each element conserved. This means that the number of atoms of each element on the reactant side of the equation must be equal to the number of atoms of each element on the product side.
Importance of Balanced Chemical Equations
Balanced chemical equations are essential in chemistry because they:
- Conserve mass: Balanced chemical equations ensure that the number of atoms of each element is conserved, following the law of conservation of mass.
- Provide a clear representation: Balanced chemical equations clearly represent the reactants and products of a chemical reaction, making it easier to understand the reaction.
- Enable calculations: Balanced chemical equations allow for calculations of quantities such as yield, limiting reagent, and percent yield.
How to Balance Chemical Equations
To balance chemical equations, follow these steps:
- Write the unbalanced equation: Write the equation with the reactants and products, without worrying about balancing.
- Count the atoms: Count the number of atoms of each element on both sides of the equation.
- Balance the elements: Balance the elements one by one, starting with elements that appear only once on each side.
- Use coefficients: Use coefficients (numbers in front of formulas of reactants or products) to balance the elements.
- Check the equation: Check the equation to ensure that it is balanced.
Example 1: Balancing a Simple Equation
Unbalanced equation: Na + Cl2 → NaCl
Step 1: Write the unbalanced equation Na + Cl2 → NaCl
Step 2: Count the atoms Na: 1, Cl: 2 (reactants), Na: 1, Cl: 1 (products)
Step 3: Balance the elements Balance Cl by multiplying NaCl by 2: Na + Cl2 → 2NaCl
Step 4: Use coefficients No coefficients needed in this case.
Step 5: Check the equation Na: 1, Cl: 2 (reactants), Na: 2, Cl: 2 (products) The equation is balanced.
Example 2: Balancing a More Complex Equation
Unbalanced equation: C3H8 + O2 → CO2 + H2O
Step 1: Write the unbalanced equation C3H8 + O2 → CO2 + H2O
Step 2: Count the atoms C: 3, H: 8, O: 2 (reactants), C: 1, H: 2, O: 3 (products)
Step 3: Balance the elements Balance C by multiplying CO2 by 3: C3H8 + O2 → 3CO2 + H2O
Balance H by multiplying H2O by 4: C3H8 + O2 → 3CO2 + 4H2O
Balance O by multiplying O2 by 5: C3H8 + 5O2 → 3CO2 + 4H2O
Step 4: Use coefficients No coefficients needed in this case.
Step 5: Check the equation C: 3, H: 8, O: 10 (reactants), C: 3, H: 8, O: 10 (products) The equation is balanced.
🔍 Note: Make sure to check your work carefully to ensure that the equation is balanced.
Conclusion
Balanced chemical equations are a fundamental concept in chemistry, and mastering them is essential for success in the field. By following the steps outlined in this worksheet, you should be able to balance simple and complex equations with ease.
What is the purpose of balancing chemical equations?
+
The purpose of balancing chemical equations is to ensure that the number of atoms of each element is conserved, following the law of conservation of mass.
How do I balance a chemical equation?
+
To balance a chemical equation, follow the steps outlined in this worksheet: write the unbalanced equation, count the atoms, balance the elements, use coefficients, and check the equation.
What is the difference between a balanced and unbalanced equation?
+
A balanced equation has the same number of atoms of each element on both the reactant and product sides, while an unbalanced equation does not.
Related Terms:
- Balancing Chemical Equations Worksheet Answers
- balancing-chemical-equations-worksheet-1-qp.pdf answers
- Easy balancing equations worksheet