Mastering Avogadro's Number Worksheet

Understanding Avogadro's Number
Avogadro’s number is a fundamental concept in chemistry, representing the number of units (atoms or molecules) in one mole of a substance. This number is crucial in calculations involving chemical reactions, stoichiometry, and atomic masses. In this worksheet, we will explore the concept of Avogadro’s number and its applications in chemistry.
What is Avogadro's Number?
Avogadro’s number, denoted by the symbol NA, is equal to 6.022 x 10^23 particles (atoms or molecules). This number is named after the Italian scientist Amedeo Avogadro, who first proposed the concept in the early 19th century. Avogadro’s number is used to convert between the amount of a substance in moles and the number of particles it contains.
Importance of Avogadro's Number
Avogadro’s number is essential in various chemical calculations, such as:
- Mole-mass conversions: Avogadro’s number helps convert between the mass of a substance in grams and the number of moles it contains.
- Chemical reactions: Avogadro’s number is used to calculate the number of particles involved in a chemical reaction, making it possible to predict the amounts of reactants and products.
- Stoichiometry: Avogadro’s number is crucial in stoichiometric calculations, which involve determining the amounts of substances required for a chemical reaction.
How to Calculate Avogadro's Number
Avogadro’s number can be calculated using the following formula:
NA = 6.022 x 10^23 particles/mol
This formula represents the number of particles (atoms or molecules) in one mole of a substance.
Worksheet Exercises
Exercise 1: Calculate the number of particles in 2 moles of carbon dioxide (CO2).
Solution: NA = 6.022 x 10^23 particles/mol Number of particles = 2 mol x 6.022 x 10^23 particles/mol = 1.2044 x 10^24 particles
Exercise 2: Calculate the mass of 1.5 moles of oxygen gas (O2) in grams.
Solution: Molar mass of O2 = 32 g/mol Mass of O2 = 1.5 mol x 32 g/mol = 48 g
Exercise 3: Calculate the number of moles of hydrogen gas (H2) required to produce 10^23 molecules of water (H2O).
Solution: 1 mole of H2 produces 2 moles of H2O Number of moles of H2 = 10^23 molecules / (2 x 6.022 x 10^23 molecules/mol) = 0.083 moles
🔍 Note: Make sure to use the correct units and significant figures when solving these exercises.
Additional Tips and Tricks
- Always use the correct units when working with Avogadro’s number (particles/mol).
- Make sure to convert between moles and particles correctly.
- Use the molar mass of a substance to convert between mass and moles.
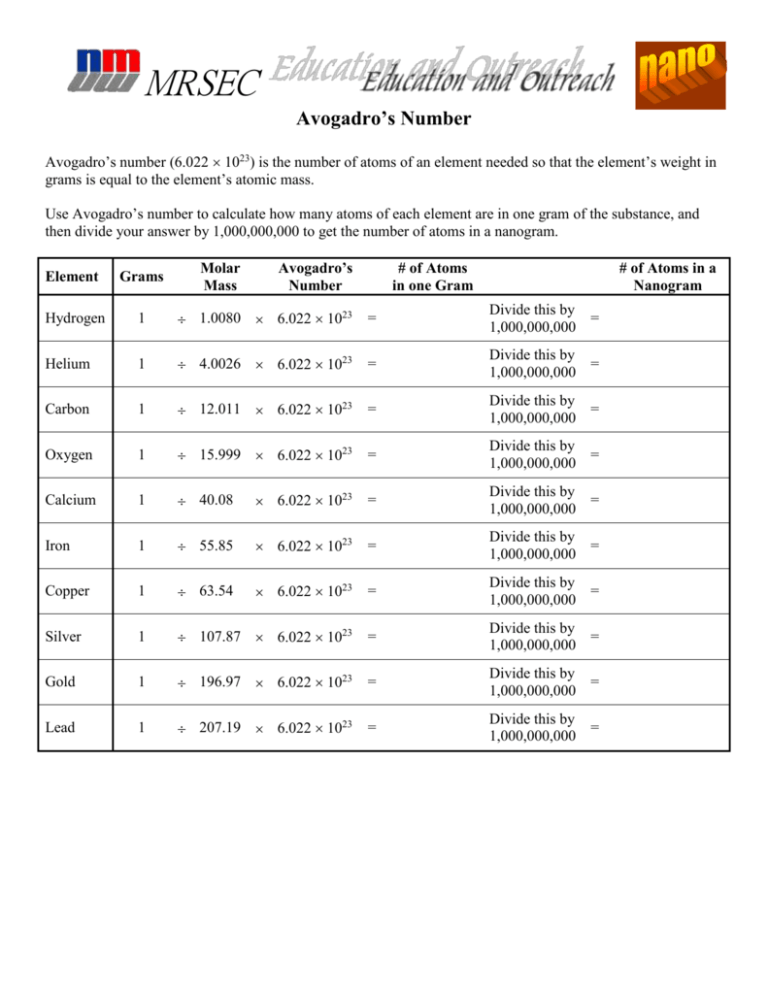
| Substance | Molar Mass (g/mol) | Number of Particles (particles/mol) |
|---|---|---|
| Carbon dioxide (CO2) | 44 g/mol | 6.022 x 10^23 particles/mol |
| Oxygen gas (O2) | 32 g/mol | 6.022 x 10^23 particles/mol |
| Hydrogen gas (H2) | 2 g/mol | 6.022 x 10^23 particles/mol |
Conclusion
In conclusion, Avogadro’s number is a fundamental concept in chemistry that represents the number of units (atoms or molecules) in one mole of a substance. By mastering Avogadro’s number, you can perform various chemical calculations, such as mole-mass conversions, chemical reactions, and stoichiometry. Practice the exercises and use the additional tips and tricks to improve your understanding of Avogadro’s number.
What is Avogadro’s number?
+Avogadro’s number is the number of units (atoms or molecules) in one mole of a substance, equal to 6.022 x 10^23 particles/mol.
Why is Avogadro’s number important in chemistry?
+Avogadro’s number is essential in various chemical calculations, such as mole-mass conversions, chemical reactions, and stoichiometry.
How can I calculate Avogadro’s number?
+Avogadro’s number can be calculated using the formula: NA = 6.022 x 10^23 particles/mol.
Related Terms:
- Avogadro s Number Worksheet
- Avogadro's number questions and answers
- Avogadro's number PDF
- Molar Mass Worksheet pdf
- The Mole Worksheet chemistry answers