Atoms and Ions Worksheet Answer Key
Atoms and Ions Worksheet Answer Key
Atoms and ions are the building blocks of matter, and understanding their properties and behavior is essential in chemistry. This worksheet will help you assess your knowledge of atoms and ions.
Section 1: Multiple Choice Questions
Choose the correct answer for each question.
- What is the smallest unit of matter that still retains the properties of an element? a) Molecule b) Compound c) Atom d) Mixture
Answer: c) Atom
- Which of the following is an example of an ion? a) Oxygen (O) b) Sodium (Na) c) Sodium ion (Na+) d) Water (H2O)
Answer: c) Sodium ion (Na+)
- What is the charge on a neutron? a) +1 b) -1 c) 0 d) +2
Answer: c) 0
Section 2: Short Answer Questions
- What is the difference between an atom and a molecule?
Answer: An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of atoms bonded together.
- What is an ion, and how is it formed?
Answer: An ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge. Ions are formed when an atom or molecule gains or loses electrons, often through chemical reactions.
Section 3: Fill-in-the-Blank Questions
- The number of protons in an atom’s nucleus determines its _______________________.
Answer: atomic number
- The number of electrons in an atom’s outermost energy level determines its _______________________.
Answer: valence
- Ions with a positive charge are called ______________________, while ions with a negative charge are called ______________________.
Answer: cations, anions
Section 4: True or False Questions
- True or False: All atoms have the same number of protons and neutrons.
Answer: False
- True or False: Ions are always formed through chemical reactions.
Answer: True
Section 5: Problems
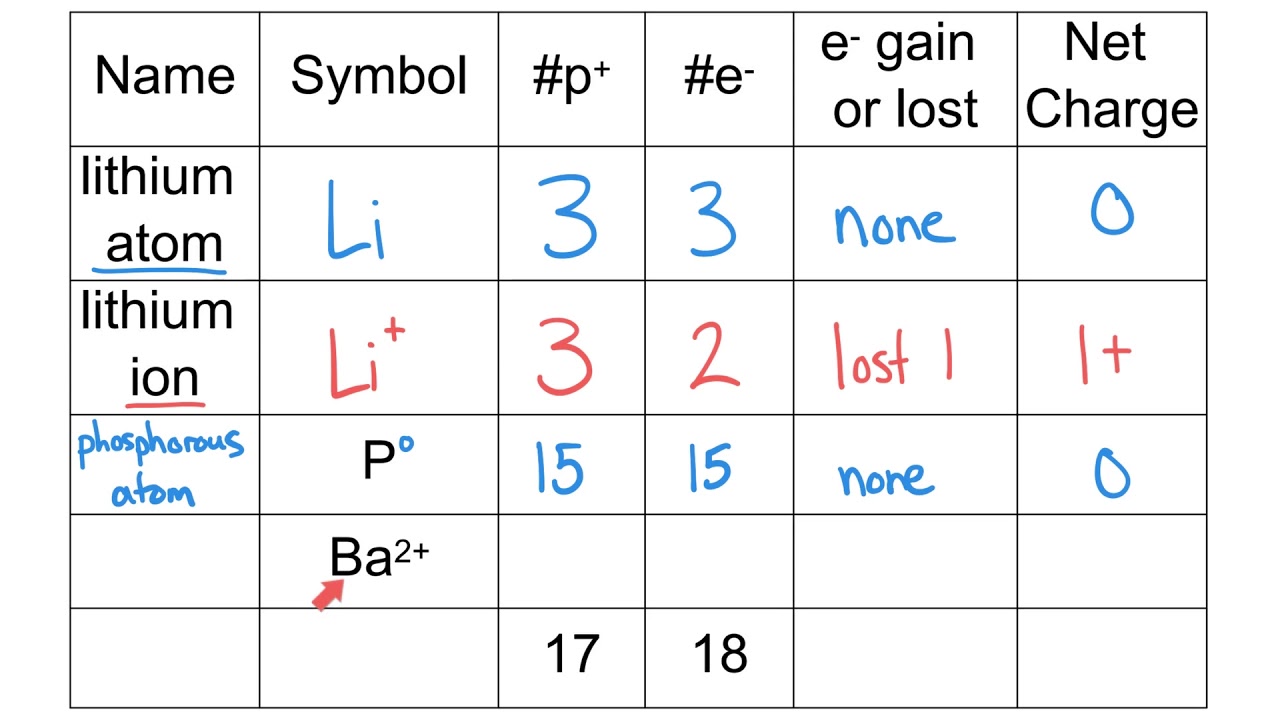
- What is the atomic number of an atom with 17 protons?
Answer: 17
- What is the charge on a sodium ion with 11 protons and 10 electrons?
Answer: +1
Notes
💡 Note: The atomic number of an element is equal to the number of protons in its atomic nucleus.
💡 Note: Ions are formed when an atom or molecule gains or loses electrons, resulting in a net positive or negative charge.
Conclusion
Atoms and ions are fundamental concepts in chemistry, and understanding their properties and behavior is essential for success in the field. By completing this worksheet, you have demonstrated your knowledge of atoms and ions and are well on your way to becoming a chemistry expert.
FAQ Section
What is the difference between an atom and a molecule?
+An atom is the smallest unit of matter that still retains the properties of an element, while a molecule is a group of atoms bonded together.
What is an ion, and how is it formed?
+An ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge. Ions are formed when an atom or molecule gains or loses electrons, often through chemical reactions.
What is the charge on a neutron?
+The charge on a neutron is 0.
Related Terms:
- Atoms and Ions Worksheet pdf
- Ions Worksheet with answers
- Isotope ion worksheet
- Ion Charges Worksheet
- Charges of Ions Worksheet pdf
- Formation of ion Worksheet