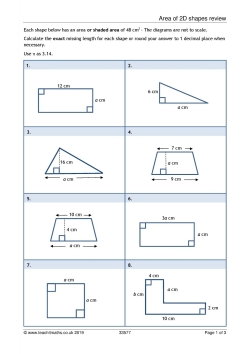
Answer Key Balancing Chemical Equations Worksheet

Mastering Chemical Equations: A Step-by-Step Guide
Chemical equations are a fundamental part of chemistry, representing the reactants, products, and sometimes the reaction conditions. However, for a chemical equation to be valid, it must be balanced, meaning the number of atoms for each element must be the same on both the reactant and product sides. In this post, we’ll delve into the world of balancing chemical equations, providing a comprehensive guide on how to tackle this essential skill.
Why Balance Chemical Equations?
Balancing chemical equations is crucial for several reasons:
- Conservation of Mass: The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. Balancing equations ensures that this law is upheld.
- Accurate Representation: A balanced equation accurately represents the reaction, allowing chemists to predict the amounts of reactants needed and products formed.
- Safety and Efficiency: In industrial settings, balanced equations help in planning and executing reactions safely and efficiently, minimizing waste and maximizing yield.
Step-by-Step Guide to Balancing Chemical Equations
Balancing a chemical equation involves a systematic approach. Here’s a step-by-step guide:
Step 1: Write the Unbalanced Equation
Start by writing the unbalanced chemical equation with the reactants on the left and the products on the right.
Step 2: Count the Atoms
Count the number of atoms for each element on both sides of the equation.
Step 3: Balance the Atoms
Begin balancing the atoms by starting with elements that appear only once on each side of the equation. Add coefficients (numbers in front of formulas of reactants or products) to balance these atoms.
Step 4: Balance Polyatomic Ions
If the equation contains polyatomic ions, balance them as a unit.
Step 5: Balance Hydrogen and Oxygen
Lastly, balance hydrogen and oxygen atoms. Hydrogen is usually balanced by adding H2O to one side, while oxygen is balanced by adding H2O and then H2 to balance the hydrogen added.
Step 6: Check the Balance
After making adjustments, re-count the atoms on both sides to ensure the equation is balanced.
Examples of Balancing Chemical Equations
Let’s apply these steps to a couple of examples:
Example 1: Combustion of Methane
Unbalanced equation: CH4 + O2 → CO2 + H2O
Balanced equation: CH4 + 2O2 → CO2 + 2H2O
Example 2: Reaction of Aluminum with Oxygen
Unbalanced equation: Al + O2 → Al2O3
Balanced equation: 4Al + 3O2 → 2Al2O3
Common Mistakes and Tips
- Change Coefficients, Not Subscripts: When balancing, change the coefficients (numbers in front of formulas) and not the subscripts (numbers within formulas).
- Balance in a Systematic Order: Start with elements that appear only once on each side and end with hydrogen and oxygen.
🔥 Note: Always double-check your work by counting the atoms on both sides after balancing the equation.
What is the primary reason for balancing chemical equations?
+The primary reason for balancing chemical equations is to adhere to the law of conservation of mass, ensuring that the number of atoms for each element is the same on both the reactant and product sides.
How do you balance polyatomic ions in a chemical equation?
+Polyatomic ions are balanced as a unit. This means adding coefficients in front of the formula of the polyatomic ion to balance it, rather than altering the subscripts within the ion.
What is the last step in balancing a chemical equation?
+The last step in balancing a chemical equation is to re-count the atoms on both sides to ensure that the equation is indeed balanced, meaning the number of atoms for each element is the same on both sides.
Mastering the art of balancing chemical equations is a fundamental skill in chemistry. By following the systematic approach outlined above, you’ll be able to balance even the most complex equations with ease. Remember, practice is key, so keep sharpening your skills with different types of equations.